Crosstalk between Metabolic Disorders and Immune Cells
- PMID: 34576181
- PMCID: PMC8469678
- DOI: 10.3390/ijms221810017
Crosstalk between Metabolic Disorders and Immune Cells
Abstract
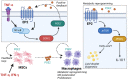
Metabolic syndrome results from multiple risk factors that arise from insulin resistance induced by abnormal fat deposition. Chronic inflammation owing to obesity primarily results from the recruitment of pro-inflammatory M1 macrophages into the adipose tissue stroma, as the adipocytes within become hypertrophied. During obesity-induced inflammation in adipose tissue, pro-inflammatory cytokines are produced by macrophages and recruit further pro-inflammatory immune cells into the adipose tissue to boost the immune response. Here, we provide an overview of the biology of macrophages in adipose tissue and the relationship between other immune cells, such as CD4+ T cells, natural killer cells, and innate lymphoid cells, and obesity and type 2 diabetes. Finally, we discuss the link between the human pathology and immune response and metabolism and further highlight potential therapeutic targets for the treatment of metabolic disorders.
Keywords: 5-aminolevulinic acid; CD4+ T cells; M1/M2 macrophages; chronic inflammation; cytokine; innate lymphoid cells; mesenchymal stem cells; natural killer cells; non-obese metabolic disorder; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance.Biochim Biophys Acta. 2014 Mar;1842(3):446-62. doi: 10.1016/j.bbadis.2013.05.017. Epub 2013 May 22. Biochim Biophys Acta. 2014. PMID: 23707515 Free PMC article. Review.
-
Regulation of metabolism by the innate immune system.Nat Rev Endocrinol. 2016 Jan;12(1):15-28. doi: 10.1038/nrendo.2015.189. Epub 2015 Nov 10. Nat Rev Endocrinol. 2016. PMID: 26553134 Review.
-
The "Big Bang" in obese fat: Events initiating obesity-induced adipose tissue inflammation.Eur J Immunol. 2015 Sep;45(9):2446-56. doi: 10.1002/eji.201545502. Epub 2015 Aug 19. Eur J Immunol. 2015. PMID: 26220361 Review.
-
The role of innate immune cells in obese adipose tissue inflammation and development of insulin resistance.Thromb Haemost. 2013 Mar;109(3):399-406. doi: 10.1160/TH12-09-0703. Epub 2013 Jan 31. Thromb Haemost. 2013. PMID: 23364297 Review.
-
Innate immune cells in the adipose tissue.Rev Endocr Metab Disord. 2018 Dec;19(4):283-292. doi: 10.1007/s11154-018-9451-6. Rev Endocr Metab Disord. 2018. PMID: 29922964 Review.
Cited by
-
miRNAs and T cell-mediated Immune Response in Disease.Yale J Biol Med. 2025 Jun 30;98(2):187-202. doi: 10.59249/PAYJ6872. eCollection 2025 Jun. Yale J Biol Med. 2025. PMID: 40589938 Free PMC article. Review.
-
Unraveling the roles of endoplasmic reticulum-associated degradation in metabolic disorders.Front Endocrinol (Lausanne). 2023 Jun 29;14:1123769. doi: 10.3389/fendo.2023.1123769. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37455916 Free PMC article. Review.
-
Associations of Placental Inflammation and Oxidative Stress Biomarkers with Glucolipid Metabolism in Children: A Birth Cohort Study in China.J Am Heart Assoc. 2024 Sep 3;13(17):e035754. doi: 10.1161/JAHA.124.035754. Epub 2024 Aug 29. J Am Heart Assoc. 2024. PMID: 39206740 Free PMC article.
-
Prostate cancer and metabolic syndrome: exploring shared signature genes through integrative analysis of bioinformatics and clinical data.Discov Oncol. 2025 May 8;16(1):698. doi: 10.1007/s12672-025-02561-9. Discov Oncol. 2025. PMID: 40338488 Free PMC article.
-
The Circadian Rhythm Regulates the Hepato-ovarian Axis Linking Polycystic Ovary Syndrome and Non-alcoholic Fatty Liver Disease.Biochem Genet. 2025 Jan 18. doi: 10.1007/s10528-024-11010-1. Online ahead of print. Biochem Genet. 2025. PMID: 39826031
References
-
- Hong E.G., Ko H.J., Cho Y.R., Kim H.J., Ma Z.X., Yu T.Y., Friedline R.H., Kurt-Jones E., Finberg R., Fischer M.A., et al. Interleukin-10 Prevents Diet-Induced Insulin Resistance by Attenuating Macrophage and Cytokine Response in Skeletal Muscle. Diabetes. 2009;58:2525–2535. doi: 10.2337/db08-1261. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

