Androgen Receptor Signaling Induces Cisplatin Resistance via Down-Regulating GULP1 Expression in Bladder Cancer
- PMID: 34576193
- PMCID: PMC8466436
- DOI: 10.3390/ijms221810030
Androgen Receptor Signaling Induces Cisplatin Resistance via Down-Regulating GULP1 Expression in Bladder Cancer
Abstract
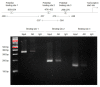
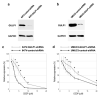
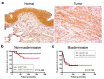
The underlying molecular mechanisms of resistance to cisplatin-based systemic chemotherapy in bladder cancer patients remain to be elucidated, while the link between androgen receptor (AR) activity and chemosensitivity in urothelial cancer has been implicated. Our DNA microarray analysis in control vs. AR knockdown bladder cancer lines identified GULP1 as a potential target of AR signaling. We herein determined the relationship between AR activity and GULP1 expression in bladder cancer cells and then assessed the functional role of GULP1 in cisplatin sensitivity. Androgen treatment in AR-positive cells or AR overexpression in AR-negative cells considerably reduced the levels of GULP1 expression. Chromatin immunoprecipitation further showed direct interaction of AR with the promoter region of GULP1. Meanwhile, GULP1 knockdown sublines were significantly more resistant to cisplatin treatment compared with respective controls. GULP1 knockdown also resulted in a significant decrease in apoptosis, as well as a significant increase in G2/M phases, when treated with cisplatin. In addition, GULP1 was immunoreactive in 74% of muscle-invasive bladder cancers from patients who had subsequently undergone neoadjuvant chemotherapy, including 53% of responders showing moderate (2+)/strong (3+) expression vs. 23% of non-responders showing 2+/3+ expression (P = 0.044). These findings indicate that GULP1 represents a key downstream effector of AR signaling in enhancing sensitivity to cisplatin treatment.
Keywords: androgen receptor; chemoresistance; cisplatin; immunohistochemistry; urothelial cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
GABBR2 as a Downstream Effector of the Androgen Receptor Induces Cisplatin Resistance in Bladder Cancer.Int J Mol Sci. 2023 Sep 6;24(18):13733. doi: 10.3390/ijms241813733. Int J Mol Sci. 2023. PMID: 37762034 Free PMC article.
-
GULP1 as a Downstream Effector of the Estrogen Receptor-β Modulates Cisplatin Sensitivity in Bladder Cancer.Cancer Genomics Proteomics. 2024 Nov-Dec;21(6):557-565. doi: 10.21873/cgp.20472. Cancer Genomics Proteomics. 2024. PMID: 39467629 Free PMC article.
-
Androgen receptor activity modulates responses to cisplatin treatment in bladder cancer.Oncotarget. 2016 Aug 2;7(31):49169-49179. doi: 10.18632/oncotarget.9994. Oncotarget. 2016. PMID: 27322140 Free PMC article.
-
Androgen receptor activation: a prospective therapeutic target for bladder cancer?Expert Opin Ther Targets. 2017 Mar;21(3):249-257. doi: 10.1080/14728222.2017.1280468. Epub 2017 Jan 19. Expert Opin Ther Targets. 2017. PMID: 28064545 Review.
-
The emerging role of the androgen receptor in bladder cancer.Endocr Relat Cancer. 2015 Oct;22(5):R265-77. doi: 10.1530/ERC-15-0209. Epub 2015 Jul 30. Endocr Relat Cancer. 2015. PMID: 26229034 Review.
Cited by
-
Multi-Omics Analysis of Novel Signature for Immunotherapy Response and Tumor Microenvironment Regulation Patterns in Urothelial Cancer.Front Cell Dev Biol. 2021 Dec 3;9:764125. doi: 10.3389/fcell.2021.764125. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34926452 Free PMC article.
-
GABBR2 as a Downstream Effector of the Androgen Receptor Induces Cisplatin Resistance in Bladder Cancer.Int J Mol Sci. 2023 Sep 6;24(18):13733. doi: 10.3390/ijms241813733. Int J Mol Sci. 2023. PMID: 37762034 Free PMC article.
-
Roles of enhancer RNAs in sex hormone-dependent cancers.J Cancer Res Clin Oncol. 2022 Feb;148(2):293-307. doi: 10.1007/s00432-021-03886-y. Epub 2022 Jan 22. J Cancer Res Clin Oncol. 2022. PMID: 35064362 Free PMC article. Review.
-
An HDAC9-associated immune-related signature predicts bladder cancer prognosis.PLoS One. 2022 Mar 3;17(3):e0264527. doi: 10.1371/journal.pone.0264527. eCollection 2022. PLoS One. 2022. PMID: 35239708 Free PMC article.
-
Biological differences underlying sex and gender disparities in bladder cancer: current synopsis and future directions.Oncogenesis. 2023 Sep 4;12(1):44. doi: 10.1038/s41389-023-00489-9. Oncogenesis. 2023. PMID: 37666817 Free PMC article. Review.
References
-
- SEER Cancer Stat Facts: Bladder Cancer. National Cancer Institute; Bethesda, MD, USA: [(accessed on 10 August 2021)]. Available online: http://seer.cancer.gov/statfacts/html/urinb.html.
-
- Rouprêt M., Babjuk M., Compérat E., Zigeuner R., Sylvester R.J., Burger M., Cowan N.C., Gontero P., Van Rhijn B.W.G., Mostafid A.H., et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2017 Update. Eur. Urol. 2018;73:111–122. doi: 10.1016/j.eururo.2017.07.036. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

