Serum Albumin: A Multifaced Enzyme
- PMID: 34576249
- PMCID: PMC8466385
- DOI: 10.3390/ijms221810086
Serum Albumin: A Multifaced Enzyme
Abstract
Human serum albumin (HSA) is the most abundant protein in plasma, contributing actively to oncotic pressure maintenance and fluid distribution between body compartments. HSA acts as the main carrier of fatty acids, recognizes metal ions, affects pharmacokinetics of many drugs, provides the metabolic modification of some ligands, renders potential toxins harmless, accounts for most of the anti-oxidant capacity of human plasma, and displays esterase, enolase, glucuronidase, and peroxidase (pseudo)-enzymatic activities. HSA-based catalysis is physiologically relevant, affecting the metabolism of endogenous and exogenous compounds including proteins, lipids, cholesterol, reactive oxygen species (ROS), and drugs. Catalytic properties of HSA are modulated by allosteric effectors, competitive inhibitors, chemical modifications, pathological conditions, and aging. HSA displays anti-oxidant properties and is critical for plasma detoxification from toxic agents and for pro-drugs activation. The enzymatic properties of HSA can be also exploited by chemical industries as a scaffold to produce libraries of catalysts with improved proficiency and stereoselectivity for water decontamination from poisonous agents and environmental contaminants, in the so called "green chemistry" field. Here, an overview of the intrinsic and metal dependent (pseudo-)enzymatic properties of HSA is reported to highlight the roles played by this multifaced protein.
Keywords: aldolase activity; anti-oxidant activity; enolase activity; enzymatic properties; esterase activity; glucuronidase activity; human serum albumin; human serum heme-albumin; peroxidase activity.
Conflict of interest statement
Authors declare no conflict of interest.
Figures

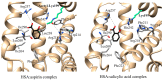
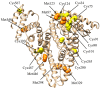
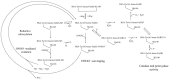
References
-
- Peters T., Jr. All about Albumin: Biochemistry, Genetics and Medical Applications. Academic Press; London, UK: San Diego, CA, USA: 1996.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

