Plasma Membrane Receptors Involved in the Binding and Response of Osteoclasts to Noncellular Components of the Bone
- PMID: 34576260
- PMCID: PMC8466431
- DOI: 10.3390/ijms221810097
Plasma Membrane Receptors Involved in the Binding and Response of Osteoclasts to Noncellular Components of the Bone
Abstract
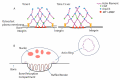
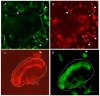
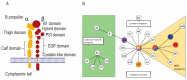
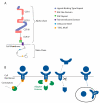
Osteoclasts differentiate from hematopoietic cells and resorb the bone in response to various signals, some of which are received directly from noncellular elements of the bone. In vitro, adherence to the bone triggers the reduction of cell-cell fusion events between osteoclasts and the activation of osteoclasts to form unusual dynamic cytoskeletal and membrane structures that are required for degrading the bone. Integrins on the surface of osteoclasts are known to receive regulatory signals from the bone matrix. Regulation of the availability of these signals is accomplished by enzymatic alterations of the bone matrix by protease activity and phosphorylation/dephosphorylation events. Other membrane receptors are present in osteoclasts and may interact with as yet unidentified signals in the bone. Bone mineral has been shown to have regulatory effects on osteoclasts, and osteoclast activity is also directly modulated by mechanical stress. As understanding of how osteoclasts and other bone cells interact with the bone has emerged, increasingly sophisticated efforts have been made to create bone biomimetics that reproduce both the structural properties of the bone and the bone's ability to regulate osteoclasts and other bone cells. A more complete understanding of the interactions between osteoclasts and the bone may lead to new strategies for the treatment of bone diseases and the production of bone biomimetics to repair defects.
Keywords: CD44; LRP1; V-ATPase; bone remodeling; extracellular vesicles; integrins; osteopontin; synaptotagmin; vacuolar H+-ATPase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

