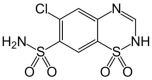
Can We Predict the Isosymmetric Phase Transition? Application of DFT Calculations to Study the Pressure Induced Transformation of Chlorothiazide
- PMID: 34576265
- PMCID: PMC8465122
- DOI: 10.3390/ijms221810100
Can We Predict the Isosymmetric Phase Transition? Application of DFT Calculations to Study the Pressure Induced Transformation of Chlorothiazide
Abstract
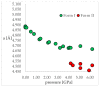
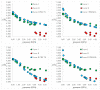
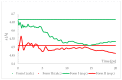
Isosymmetric structural phase transition (IPT, type 0), in which there are no changes in the occupation of Wyckoff positions, the number of atoms in the unit cell, and the space group symmetry, is relatively uncommon. Chlorothiazide, a diuretic agent with a secondary function as an antihypertensive, has been proven to undergo pressure-induced IPT of Form I to Form II at 4.2 GPa. For that reason, it has been chosen as a model compound in this study to determine if IPT can be predicted in silico using periodic DFT calculations. The transformation of Form II into Form I, occurring under decompression, was observed in geometry optimization calculations. However, the reverse transition was not detected, although the calculated differences in the DFT energies and thermodynamic parameters indicated that Form II should be more stable at increased pressure. Finally, the IPT was successfully simulated using ab initio molecular dynamics calculations.
Keywords: CASTEP; DFT; ab initio molecular dynamics; aiMD; phase transition; polymorphism.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

