Actions and Roles of FSH in Germinative Cells
- PMID: 34576272
- PMCID: PMC8470522
- DOI: 10.3390/ijms221810110
Actions and Roles of FSH in Germinative Cells
Abstract
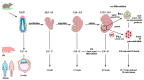
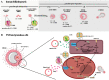
Follicle stimulating hormone (FSH) is produced by the pituitary gland in a coordinated hypothalamic-pituitary-gonadal (HPG) axis event, plays important roles in reproduction and germ cell development during different phases of reproductive development (fetal, neonatal, puberty, and adult life), and is consequently essential for fertility. FSH is a heterodimeric glycoprotein hormone of two dissociable subunits, α and β. The FSH β-subunit (FSHβ) function starts upon coupling to its specific receptor: follicle-stimulating hormone receptor (FSHR). FSHRs are localized mainly on the surface of target cells on the testis and ovary (granulosa and Sertoli cells) and have recently been found in testicular stem cells and extra-gonadal tissue. Several reproduction disorders are associated with absent or low FSH secretion, with mutation of the FSH β-subunit or the FSH receptor, and/or its signaling pathways. However, the influence of FSH on germ cells is still poorly understood; some studies have suggested that this hormone also plays a determinant role in the self-renewal of germinative cells and acts to increase undifferentiated spermatogonia proliferation. In addition, in vitro, together with other factors, it assists the process of differentiation of primordial germ cells (PGCLCs) into gametes (oocyte-like and SSCLCs). In this review, we describe relevant research on the influence of FSH on spermatogenesis and folliculogenesis, mainly in the germ cell of humans and other species. The possible roles of FSH in germ cell generation in vitro are also presented.
Keywords: germ cell line; gonadotrophin; reproduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures