Oxidative Folding of Proteins: The "Smoking Gun" of Glutathione
- PMID: 34576311
- PMCID: PMC8468038
- DOI: 10.3390/ijms221810148
Oxidative Folding of Proteins: The "Smoking Gun" of Glutathione
Abstract
Glutathione has long been suspected to be the primary low molecular weight compound present in all cells promoting the oxidative protein folding, but twenty years ago it was found "not guilty". Now, new surprising evidence repeats its request to be the "smoking gun" which reopens the criminal trial revealing the crucial involvement of this tripeptide.
Keywords: cysteine reactivity; glutathione; glutathionylation; nitrosylation; oxidative folding; ribosomal exit tunnel; transient complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

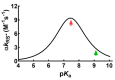

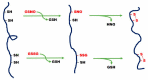
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

