Monobac System-A Single Baculovirus for the Production of rAAV
- PMID: 34576695
- PMCID: PMC8465638
- DOI: 10.3390/microorganisms9091799
Monobac System-A Single Baculovirus for the Production of rAAV
Abstract
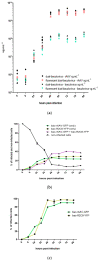
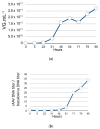
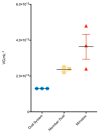
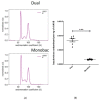
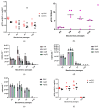
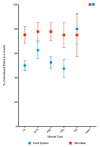
Large-scale manufacturing of rAAV is a bottleneck for the development of genetic disease treatments. The baculovirus/Sf9 cell system underpins the first rAAV treatment approved by EMA and remains one of the most advanced platforms for rAAV manufacturing. Despite early successes, rAAV is still a complex biomaterial to produce. Efficient production of the recombinant viral vector requires that AAV replicase and capsid genes be co-located with the recombinant AAV genome. Here, we present the Monobac system, a singular, modified baculovirus genome that contains all of these functions. To assess the relative yields between the dual baculovirus and Monobac systems, we prepared each system with a transgene encoding γSGC and evaluated vectors' potency in vivo. Our results show that rAAV production using the Monobac system not only yields higher titers of rAAV vector but also a lower amount of DNA contamination from baculovirus.
Keywords: baculovirus; gene therapy; rAAV.
Conflict of interest statement
L.G., A.J. and O.-W.M. are inventors of patent WO/2013/014400. The funder provided support in the form of salaries for all authors, but did not have any additional role in the study design, data collection and analysis or preparation of the manuscript.
Figures


References
-
- Alazard-Dany N., Nicolas A., Ploquin A., Strasser R., Greco A., Epstein A.L., Fraefel C., Salvetti A. Definition of herpes simplex virus type 1 helper activities for adeno-associated virus early replication events. PLoS Pathog. 2009;5:e1000340. doi: 10.1371/journal.ppat.1000340. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

