Planktonic and Biofilm-Associated Pseudomonas aeruginosa and Staphylococcus epidermidis Elicit Differential Human Peripheral Blood Cell Responses
- PMID: 34576742
- PMCID: PMC8470397
- DOI: 10.3390/microorganisms9091846
Planktonic and Biofilm-Associated Pseudomonas aeruginosa and Staphylococcus epidermidis Elicit Differential Human Peripheral Blood Cell Responses
Abstract
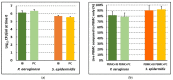
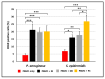
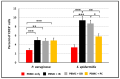
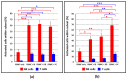
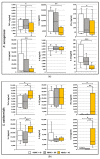
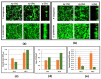
Despite the considerable progress made in recent years, our understanding of the human immune response to microbial biofilms is still poor. The aim of the present study was to compare the in vitro response of human peripheral blood mononuclear cells (PBMC) to biofilms and planktonic cells of Pseudomonas aeruginosa and Staphylococcus epidermidis, two bacterial species particularly relevant in patients with cystic fibrosis or undergoing endovascular catheterization, respectively. PBMC isolated from healthy donors were co-cultured with 24 h-old biofilms or with exponentially growing cells of both species. Following 24 h of co-culture, the expression of early activation markers and the levels of cytokines in the culture supernatants were assessed by flow cytometry, while biofilm biomass and architecture were evaluated by crystal violet staining, CFU count, and confocal microscopy. Around 20% of PBMC was activated in response to both biofilms and planktonic cells of P. aeruginosa. In contrast, planktonic cells of S. epidermidis induced a statistically higher degree of activation than their biofilm counterpart (25% versus 15%; p < 0.01). P. aeruginosa biofilms stimulated pro-inflammatory (TNF-α, IL-1β, IFN-γ, and IL-6) and anti-inflammatory (IL-10) cytokine production at statistically significant levels higher than its planktonic counterpart, while an opposite trend was observed with S. epidermidis. Differences in the architecture of the biofilms and in the number of PBMC infiltrating the biofilms between the two bacterial species may at least partially explain these findings. Collectively, the results obtained highlighted marked differences in the host-cell response depending on the species and the mode of growth (biofilms versus planktonic cultures), allowing speculations on the different strategies adopted by P. aeruginosa and S. epidermidis to persist in the host during the course of chronic infections.
Keywords: Pseudomonas aeruginosa; Staphylococcus epidermidis; biofilm; immune response; peripheral blood mononuclear cells; planktonic cells.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses and interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

