Sensitivity of Rapid Antigen Testing and RT-PCR Performed on Nasopharyngeal Swabs versus Saliva Samples in COVID-19 Hospitalized Patients: Results of a Prospective Comparative Trial (RESTART)
- PMID: 34576805
- PMCID: PMC8464722
- DOI: 10.3390/microorganisms9091910
Sensitivity of Rapid Antigen Testing and RT-PCR Performed on Nasopharyngeal Swabs versus Saliva Samples in COVID-19 Hospitalized Patients: Results of a Prospective Comparative Trial (RESTART)
Abstract
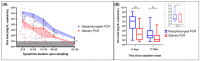
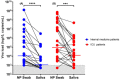
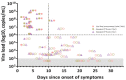
Saliva sampling could serve as an alternative non-invasive sample for SARS-CoV-2 diagnosis while rapid antigen tests (RATs) might help to mitigate the shortage of reagents sporadically encountered with RT-PCR. Thus, in the RESTART study we compared antigen and RT-PCR testing methods on nasopharyngeal (NP) swabs and salivary samples. We conducted a prospective observational study among COVID-19 hospitalized patients between 10 December 2020 and 1 February 2021. Paired saliva and NP samples were investigated by RT-PCR (Cobas 6800, Roche-Switzerland, Basel, Switzerland) and by two rapid antigen tests: One Step Immunoassay Exdia® COVID-19 Ag (Precision Biosensor, Daejeon, Korea) and Standard Q® COVID-19 Rapid Antigen Test (Roche-Switzerland). A total of 58 paired NP-saliva specimens were collected. A total of 32 of 58 (55%) patients were hospitalized in the intensive care unit, and the median duration of symptoms was 11 days (IQR 5-19). NP and salivary RT-PCR exhibited sensitivity of 98% and 69% respectively, whereas the specificity of these RT-PCRs assays was 100%. The NP RATs exhibited much lower diagnostic performance, with sensitivities of 35% and 41% for the Standard Q® and Exdia® assays, respectively, when a wet-swab approach was used (i.e., when the swab was diluted in the viral transport medium (VTM) before testing). The sensitivity of the dry-swab approach was slightly better (47%). These antigen tests exhibited very low sensitivity (4% and 8%) when applied to salivary swabs. Nasopharyngeal RT-PCR is the most accurate test for COVID-19 diagnosis in hospitalized patients. RT-PCR on salivary samples may be used when nasopharyngeal swabs are contraindicated. RATs are not appropriate for hospitalized patients.
Keywords: RT-PCR; SARS-CoV-2 diagnosis; rapid antigen testing; saliva; viral transport medium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- WHO Diagnostic Testing for SARS-CoV-2. [(accessed on 7 April 2021)]. Available online: https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2.
-
- Butler-Laporte G., Lawandi A., Schiller I., Yao M., Dendukuri N., McDonald E.G., Lee T.C. Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2021;181:353–358. doi: 10.1001/jamainternmed.2020.8876. - DOI - PMC - PubMed
-
- Jamal A.J., Mozafarihashjin M., Coomes E., Powis J., Li A.X., Paterson A., Anceva-Sami S., Barati S., Crowl G., Faheem A., et al. Sensitivity of nasopharyngeal swabs and saliva for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;72:1064–1066. doi: 10.1093/cid/ciaa848. - DOI - PMC - PubMed
-
- Pasomsub E., Watcharananan S.P., Boonyawat K., Janchompoo P., Wongtabtim G., Suksuwan W., Sungkanuparph S., Phuphuakrat A. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: A cross-sectional study. Clin. Microbiol. Infect. 2021;27:285.e1–285.e4. doi: 10.1016/j.cmi.2020.05.001. - DOI - PMC - PubMed
-
- Schwob J.-M., Miauton A., Petrovic D., Perdrix J., Senn N., Jaton K., Opota O., Maillard A., Minghelli G., Cornuz J., et al. Antigen rapid tests, nasopharyngeal PCR and saliva PCR to detect SARS-CoV-2: A prospective comparative clinical trial. [(accessed on 7 April 2021)];medXriv. 2020 Available online: https://www.medrxiv.org/content/10.1101/2020.11.23.20237057v1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

