Wolf in Sheep's Clothing: Clostridioides difficile Biofilm as a Reservoir for Recurrent Infections
- PMID: 34576818
- PMCID: PMC8470499
- DOI: 10.3390/microorganisms9091922
Wolf in Sheep's Clothing: Clostridioides difficile Biofilm as a Reservoir for Recurrent Infections
Abstract
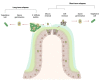
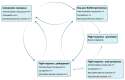
The microbiota inhabiting the intestinal tract provide several critical functions to its host. Microorganisms found at the mucosal layer form organized three-dimensional structures which are considered to be biofilms. Their development and functions are influenced by host factors, host-microbe interactions, and microbe-microbe interactions. These structures can dictate the health of their host by strengthening the natural defenses of the gut epithelium or cause disease by exacerbating underlying conditions. Biofilm communities can also block the establishment of pathogens and prevent infectious diseases. Although these biofilms are important for colonization resistance, new data provide evidence that gut biofilms can act as a reservoir for pathogens such as Clostridioides difficile. In this review, we will look at the biofilms of the intestinal tract, their contribution to health and disease, and the factors influencing their formation. We will then focus on the factors contributing to biofilm formation in C. difficile, how these biofilms are formed, and their properties. In the last section, we will look at how the gut microbiota and the gut biofilm influence C. difficile biofilm formation, persistence, and transmission.
Keywords: CDI relapsing; Clostridioides difficile infection; biofilm inducers; colonisation resistance; commensal microbiota; dysbiosis; mucosal-biofilm; persistence.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Medical

