Titanium Wear Particles Exacerbate S. epidermidis-Induced Implant-Related Osteolysis and Decrease Efficacy of Antibiotic Therapy
- PMID: 34576840
- PMCID: PMC8468325
- DOI: 10.3390/microorganisms9091945
Titanium Wear Particles Exacerbate S. epidermidis-Induced Implant-Related Osteolysis and Decrease Efficacy of Antibiotic Therapy
Abstract
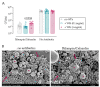
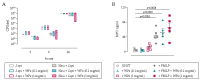
Total joint arthroplasty (TJA) surgeries are common orthopedic procedures, but bacterial infection remains a concern. The aim of this study was to assess interactions between wear particles (WPs) and immune cells in vitro and to investigate if WPs affect the severity, or response to antibiotic therapy, of a Staphylococcus epidermidis orthopedic device-related infection (ODRI) in a rodent model. Biofilms grown on WPs were challenged with rifampin and cefazolin (100 µg/mL) to determine antibiotic efficacy. Neutrophils or peripheral blood mononuclear cells (PBMCs) were incubated with or without S. epidermidis and WPs, and myeloperoxidase (MPO) and cytokine release were analyzed, respectively. In the ODRI rodent model, rats (n = 36) had a sterile or S. epidermidis-inoculated screw implanted in the presence or absence of WPs, and a subgroup was treated with antibiotics. Bone changes were monitored using microCT scanning. The presence of WPs decreased antibiotic efficacy against biofilm-resident bacteria and promoted MPO and pro-inflammatory cytokine production in vitro. WPs exacerbated osteolytic responses to S. epidermidis infection and markedly reduced antibiotic efficacy in vivo. Overall, this work shows that the presence of titanium WPs reduces antibiotic efficacy in vitro and in vivo, induces proinflammatory cytokine release, and exacerbates S. epidermidis-induced osteolysis.
Keywords: Staphylococcus epidermidis; antibiotics; microCT; osteomyelitis; wear particles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

