Antipruritic Effect of Nalbuphine, a Kappa Opioid Receptor Agonist, in Mice: A Pan Antipruritic
- PMID: 34576988
- PMCID: PMC8466557
- DOI: 10.3390/molecules26185517
Antipruritic Effect of Nalbuphine, a Kappa Opioid Receptor Agonist, in Mice: A Pan Antipruritic
Abstract
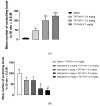
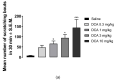
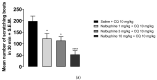
Antipruritic effects of kappa opioid receptor (KOR) agonists have been shown in rodent models of acute and chronic scratching (itchlike behavior). Three KOR agonists, nalfurafine, difelikefalin, and nalbuphine, are in clinical studies for antipruritic effects in chronic itch of systemic and skin diseases. Nalfurafine (in Japan) and difelikefalin (in the USA) were approved to be used in the treatment of chronic itch in hemodialysis patients. The FDA-approved nalbuphine has been used in clinic for over 40 years, and it is the only narcotic agonist that is not scheduled. We aimed to study (a) antiscratch activity of nalbuphine against TAT-HIV-1 protein (controls HIV transcription)-, deoxycholic acid (DCA, bile acid)-, and chloroquine (CQ)-induced scratching in a mouse model of acute itch; and (b) whether the effect of nalbuphine is produced via KORs. First, dose-responses were developed for pruritogens. Mice were pretreated with nalbuphine (0.3-10 mg/kg) and then a submaximal dose of pruritogens were administered and the number of scratching bouts was counted. To study if the antiscratch effect of nalbuphine is produced via KOR, we used KOR knock out mice and pharmacologic inhibition of KORs using nor-binaltorphimine, a KOR antagonist. For this aim, we used CQ as a pruritogen. We found that: (a) TAT-HIV-1 protein elicits scratching in a dose-dependent manner; (b) nalbuphine inhibits scratching induced by TAT-HIV-1, DCA, and CQ dose-dependently; and (c) nalbuphine inhibits scratching induced by CQ through KORs. In conclusion, nalbuphine inhibits scratching elicited by multiple pruritogens.
Keywords: TAT-HIV; chloroquine; cholestasis; deoxycholic acid; kappa opioid receptor agonist; mice; nalbuphine; pruritis; scratching.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Antipruritic Effects of Kappa Opioid Receptor Agonists: Evidence from Rodents to Humans.Handb Exp Pharmacol. 2022;271:275-292. doi: 10.1007/164_2020_420. Handb Exp Pharmacol. 2022. PMID: 33296031
-
Nalfurafine prevents 5'-guanidinonaltrindole- and compound 48/80-induced spinal c-fos expression and attenuates 5'-guanidinonaltrindole-elicited scratching behavior in mice.Neuroscience. 2009 Sep 29;163(1):23-33. doi: 10.1016/j.neuroscience.2009.06.016. Epub 2009 Jun 10. Neuroscience. 2009. PMID: 19524022 Free PMC article.
-
Nalbuphine, a kappa opioid receptor agonist and mu opioid receptor antagonist attenuates pruritus, decreases IL-31, and increases IL-10 in mice with contact dermatitis.Eur J Pharmacol. 2019 Dec 1;864:172702. doi: 10.1016/j.ejphar.2019.172702. Epub 2019 Sep 27. Eur J Pharmacol. 2019. PMID: 31568781 Free PMC article.
-
Targeting Itch with Ligands Selective for κ Opioid Receptors.Handb Exp Pharmacol. 2015;226:291-314. doi: 10.1007/978-3-662-44605-8_16. Handb Exp Pharmacol. 2015. PMID: 25861786 Review.
-
Systemic kappa opioid receptor agonists in the treatment of chronic pruritus: a literature review.Acta Derm Venereol. 2012 Sep;92(5):555-60. doi: 10.2340/00015555-1353. Acta Derm Venereol. 2012. PMID: 22504709 Review.
Cited by
-
Discovery of a Small Molecule Activator of Slack (Kcnt1) Potassium Channels That Significantly Reduces Scratching in Mouse Models of Histamine-Independent and Chronic Itch.Adv Sci (Weinh). 2024 Apr;11(15):e2307237. doi: 10.1002/advs.202307237. Epub 2024 Feb 13. Adv Sci (Weinh). 2024. PMID: 38350720 Free PMC article.
-
Genetic Variation A118G in the OPRM1 Gene Underlies the Dimorphic Response to Epidural Opioid-Induced Itch.Neurosci Bull. 2025 May 17. doi: 10.1007/s12264-025-01411-6. Online ahead of print. Neurosci Bull. 2025. PMID: 40381142
-
Opioidergic Signaling-A Neglected, Yet Potentially Important Player in Atopic Dermatitis.Int J Mol Sci. 2022 Apr 8;23(8):4140. doi: 10.3390/ijms23084140. Int J Mol Sci. 2022. PMID: 35456955 Free PMC article. Review.
-
Difelikefalin in the Treatment of Chronic Kidney Disease-Associated Pruritus: A Systematic Review.Pharmaceuticals (Basel). 2022 Jul 28;15(8):934. doi: 10.3390/ph15080934. Pharmaceuticals (Basel). 2022. PMID: 36015082 Free PMC article. Review.
-
Limitations and potential of κOR biased agonists for pain and itch management.Neuropharmacology. 2024 Nov 1;258:110061. doi: 10.1016/j.neuropharm.2024.110061. Epub 2024 Jul 2. Neuropharmacology. 2024. PMID: 38960136 Review.
References
-
- Kamimura K., Yokoo T., Kamimura H., Sakamaki A., Abe S., Tsuchiya A., Takamura M., Kawai H., Yamagiwa S., Terai S. Long-term efficacy and safety of nalfurafine hydrochloride on pruritus in chronic liver disease patients: Patient-reported outcome based analyses. PLoS ONE. 2017;12:e0178991. doi: 10.1371/journal.pone.0178991. - DOI - PMC - PubMed
-
- Yagi M., Tanaka A., Namisaki T., Takahashi A., Abe M., Honda A., Matsuzaki Y., Ohira H., Yoshiji H., Takikawa H. Is patient-reported outcome improved by nalfurafine hydrochloride in patients with primary biliary cholangitis and refractory pruritus? A post-marketing, single-arm, prospective study. J. Gastroenterol. 2018;53:1151–1158. doi: 10.1007/s00535-018-1465-z. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

