N-Heterocyclic Carbene Iron Complexes as Anticancer Agents: In Vitro and In Vivo Biological Studies
- PMID: 34577006
- PMCID: PMC8470334
- DOI: 10.3390/molecules26185535
N-Heterocyclic Carbene Iron Complexes as Anticancer Agents: In Vitro and In Vivo Biological Studies
Abstract
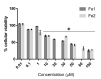
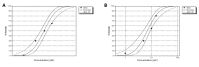
Cisplatin and its derivatives are commonly used in chemotherapeutic treatments of cancer, even though they suffer from many toxic side effects. The problems that emerge from the use of these metal compounds led to the search for new complexes capable to overcome the toxic side effects. Here, we report the evaluation of the antiproliferative activity of Fe(II) cyclopentadienyl complexes bearing n-heterocyclic carbene ligands in tumour cells and their in vivo toxicological profile. The in vitro antiproliferative assays demonstrated that complex Fe1 displays the highest cytotoxic activity both in human colorectal carcinoma cells (HCT116) and ovarian carcinoma cells (A2780) with IC50 values in the low micromolar range. The antiproliferative effect of Fe1 was even higher than cisplatin. Interestingly, Fe1 showed low in vivo toxicity, and in vivo analyses of Fe1 and Fe2 compounds using colorectal HCT116 zebrafish xenograft showed that both reduce the proliferation of human HCT116 colorectal cancer cells in vivo.
Keywords: N-heterocyclic carbene; anticancer activity; iron(II)–NHC complexes; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
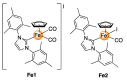

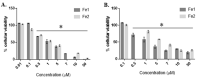



Similar articles
-
The anticancer activity of an air-stable Pd(I)-NHC (NHC = N-heterocyclic carbene) dimer.Chem Commun (Camb). 2020 Oct 13;56(81):12238-12241. doi: 10.1039/d0cc03883k. Chem Commun (Camb). 2020. PMID: 32926011
-
Anticancer Gold N-Heterocyclic Carbene Complexes: A Comparative in vitro and ex vivo Study.ChemMedChem. 2017 Sep 7;12(17):1429-1435. doi: 10.1002/cmdc.201700316. Epub 2017 Aug 29. ChemMedChem. 2017. PMID: 28741878
-
Synthesis and in-depth studies on the anticancer activity of novel palladacyclopentadienyl complexes stabilized by N-Heterocyclic carbene ligands.Eur J Med Chem. 2019 Oct 1;179:325-334. doi: 10.1016/j.ejmech.2019.06.065. Epub 2019 Jun 22. Eur J Med Chem. 2019. PMID: 31255929
-
N-heterocyclic carbene metal complexes as bio-organometallic antimicrobial and anticancer drugs.Future Med Chem. 2015;7(10):1305-33. doi: 10.4155/fmc.15.61. Future Med Chem. 2015. PMID: 26144266 Review.
-
New insights in Au-NHCs complexes as anticancer agents.Eur J Med Chem. 2018 Feb 25;146:709-746. doi: 10.1016/j.ejmech.2018.01.065. Epub 2018 Jan 31. Eur J Med Chem. 2018. PMID: 29407992 Review.
Cited by
-
Insight on cytotoxic NHC gold(I) halide complexes evaluated in multifaceted culture systems.Curr Res Toxicol. 2024 May 23;6:100174. doi: 10.1016/j.crtox.2024.100174. eCollection 2024. Curr Res Toxicol. 2024. PMID: 38841651 Free PMC article.
-
Synthesis, Structural Characterization, Cytotoxicity, and Protein/DNA Binding Properties of Pyridoxylidene-Aminoguanidine-Metal (Fe, Co, Zn, Cu) Complexes.Int J Mol Sci. 2023 Sep 29;24(19):14745. doi: 10.3390/ijms241914745. Int J Mol Sci. 2023. PMID: 37834192 Free PMC article.
-
2D and 3D anticancer activity of diiron bis-cyclopentadienyl complexes incorporating flurbiprofen and chlorambucil.RSC Med Chem. 2025 May 6. doi: 10.1039/d4md01011f. Online ahead of print. RSC Med Chem. 2025. PMID: 40734663 Free PMC article.
-
A Benzimidazole-Based N-Heterocyclic Carbene Derivative Exhibits Potent Antiproliferative and Apoptotic Effects against Colorectal Cancer.Medicina (Kaunas). 2024 Aug 23;60(9):1379. doi: 10.3390/medicina60091379. Medicina (Kaunas). 2024. PMID: 39336420 Free PMC article.
-
Heterocycles in Breast Cancer Treatment: The Use of Pyrazole Derivatives.Curr Med Chem. 2023;30(10):1145-1174. doi: 10.2174/0929867329666220829091830. Curr Med Chem. 2023. PMID: 36043746 Free PMC article. Review.
References
-
- Pedrosa P., Carvalho A., Baptista P.V., Fernandes A.R. Basic Concepts Viewed from Frontier in Inorganic Coordination Chemistry. Intech. Open; London, UK: 2018. Inorganic Coordination Chemistry: Where We Stand in Cancer Treatment? - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

