Convenient Synthesis of Pyrazolo[4',3':5,6]pyrano[4,3- c][1,2]oxazoles via Intramolecular Nitrile Oxide Cycloaddition
- PMID: 34577075
- PMCID: PMC8469150
- DOI: 10.3390/molecules26185604
Convenient Synthesis of Pyrazolo[4',3':5,6]pyrano[4,3- c][1,2]oxazoles via Intramolecular Nitrile Oxide Cycloaddition
Abstract
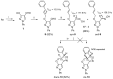
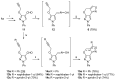
A simple and efficient synthetic route to the novel 3a,4-dihydro-3H,7H- and 4H,7H-pyrazolo[4',3':5,6]pyrano[4,3-c][1,2]oxazole ring systems from 3-(prop-2-en-1-yloxy)- or 3-(prop-2-yn-1-yloxy)-1H-pyrazole-4-carbaldehyde oximes has been developed by employing the intramolecular nitrile oxide cycloaddition (INOC) reaction as the key step. The configuration of intermediate aldoximes was unambiguously determined using NOESY experimental data and comparison of the magnitudes of 1JCH coupling constants of the iminyl moiety, which were greater by approximately 13 Hz for the predominant syn isomer. The structures of the obtained heterocyclic products were confirmed by detailed 1H, 13C and 15N NMR spectroscopic experiments and HRMS measurements.
Keywords: 4-pyrazolaldoximes 1JCH; fused ring systems; intramolecular nitrile oxide cycloaddition; isoxazole 15N NMR; isoxazoline 15N NMR; isoxazoline/isoxazole; pyrazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A convenient approach to heterocyclic building blocks: synthesis of novel ring systems containing a [5,6]Pyrano[2,3-c]pyrazol-4(1H)-one moiety.Molecules. 2007 Jan 26;12(1):60-73. doi: 10.3390/12010060. Molecules. 2007. PMID: 17693953 Free PMC article.
-
Iminosugar C-Nitromethyl Glycoside: Stereoselective Synthesis of Isoxazoline and Isoxazole-Fused Bicyclic Iminosugars.J Org Chem. 2018 Feb 2;83(3):1558-1564. doi: 10.1021/acs.joc.7b02803. Epub 2018 Jan 24. J Org Chem. 2018. PMID: 29313687
-
Highly regio- and diastereoselective, acidic clay supported intramolecular nitrile oxide-alkene cycloaddition on D-ribose derived nitriles: an efficient synthetic route to isoxazoline fused five and six membered carbocycles.Carbohydr Res. 2014 Oct 29;398:13-8. doi: 10.1016/j.carres.2014.06.007. Epub 2014 Jun 19. Carbohydr Res. 2014. PMID: 25238125
-
Synthesis of Bicyclic Isoxazoles and Isoxazolines via Intramolecular Nitrile Oxide Cycloaddition.Molecules. 2015 Jun 12;20(6):10910-27. doi: 10.3390/molecules200610910. Molecules. 2015. PMID: 26076111 Free PMC article.
-
Recyclization of 5-Amino- oxazoles as a Route to new Functionalized Heterocycles (Developments of V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the NAS of Ukraine).Chem Rec. 2024 Feb;24(2):e202300264. doi: 10.1002/tcr.202300264. Epub 2023 Oct 26. Chem Rec. 2024. PMID: 37882374 Review.
Cited by
-
Special Issue "Recent Advances in the Synthesis, Functionalization and Applications of Pyrazole-Type Compounds II".Molecules. 2023 Aug 4;28(15):5873. doi: 10.3390/molecules28155873. Molecules. 2023. PMID: 37570842 Free PMC article.
-
Synthesis and Characterization of Novel Heterocyclic Chalcones from 1-Phenyl-1H-pyrazol-3-ol.Molecules. 2022 Jun 10;27(12):3752. doi: 10.3390/molecules27123752. Molecules. 2022. PMID: 35744875 Free PMC article.
-
On the Question of Zwitterionic Intermediates in the [3+2] Cycloaddition Reactions between Aryl Azides and Ethyl Propiolate.Molecules. 2023 Dec 18;28(24):8152. doi: 10.3390/molecules28248152. Molecules. 2023. PMID: 38138640 Free PMC article.
-
Pyrazole-based lamellarin O analogues: synthesis, biological evaluation and structure-activity relationships.RSC Adv. 2023 Mar 10;13(12):7897-7912. doi: 10.1039/d3ra00972f. eCollection 2023 Mar 8. RSC Adv. 2023. PMID: 36909769 Free PMC article.
-
Synthesis and Antiproliferative Activity of 2,4,6,7-Tetrasubstituted-2H-pyrazolo[4,3-c]pyridines.Molecules. 2021 Nov 8;26(21):6747. doi: 10.3390/molecules26216747. Molecules. 2021. PMID: 34771163 Free PMC article.
References
-
- Browder C.C. Recent Advances in Intramolecular Nitrile Oxide Cycloadditions in the Synthesis of 2-Isoxazolines. Curr. Org. Synth. 2011;8:628–644. doi: 10.2174/157017911796957393. - DOI
-
- Roscales S., Plumet J. Mini-Review: Organic Catalysts in the 1,3-Dipolar Cycloaddition Reactions of Nitrile Oxides. Heterocycles. 2019;99:725–744. doi: 10.3987/REV-18-SR(F)4. - DOI
-
- Huisgen R. Cycloadditions—Definition, Classification, and Characterization. Angew. Chem. Int. Ed. Engl. 1968;7:321–328. doi: 10.1002/ange.19680800902. - DOI
-
- Padwa A. Intramolecular 1,3-Dipolar Cycloaddition Reactions. Angew. Chem. Int. Ed. Engl. 1976;15:123–136. doi: 10.1002/anie.197601231. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

