Ezrin Modulates the Cell Surface Expression of Programmed Cell Death Ligand-1 in Human Cervical Adenocarcinoma Cells
- PMID: 34577118
- PMCID: PMC8469114
- DOI: 10.3390/molecules26185648
Ezrin Modulates the Cell Surface Expression of Programmed Cell Death Ligand-1 in Human Cervical Adenocarcinoma Cells
Abstract
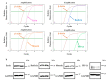
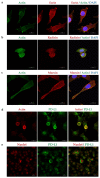
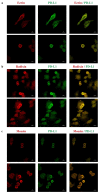
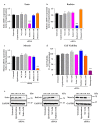
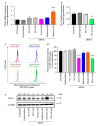
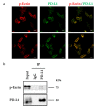
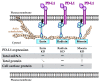
Cancer cells employ programmed cell death ligand-1 (PD-L1), an immune checkpoint protein that binds to programmed cell death-1 (PD-1) and is highly expressed in various cancers, including cervical carcinoma, to abolish T-cell-mediated immunosurveillance. Despite a key role of PD-L1 in various cancer cell types, the regulatory mechanism for PD-L1 expression is largely unknown. Understanding this mechanism could provide a novel strategy for cervical cancer therapy. Here, we investigated the influence of ezrin/radixin/moesin (ERM) family scaffold proteins, crosslinking the actin cytoskeleton and certain plasma membrane proteins, on the expression of PD-L1 in HeLa cells. Our results showed that all proteins were expressed at mRNA and protein levels and that all ERM proteins were highly colocalized with PD-L1 in the plasma membrane. Interestingly, immunoprecipitation assay results demonstrated that PD-L1 interacted with ERM as well as actin cytoskeleton proteins. Furthermore, gene silencing of ezrin, but not radixin and moesin, remarkably decreased the protein expression of PD-L1 without affecting its mRNA expression. In conclusion, ezrin may function as a scaffold protein for PD-L1; regulate PD-L1 protein expression, possibly via post-translational modification in HeLa cells; and serve as a potential therapeutic target for cervical cancer, improving the current immune checkpoint blockade therapy.
Keywords: cervical cancer; ezrin/radixin/moesin; immune check point inhibitor; programmed cell death ligand-1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Moesin Serves as Scaffold Protein for PD-L1 in Human Uterine Cervical Squamous Carcinoma Cells.J Clin Med. 2022 Jul 1;11(13):3830. doi: 10.3390/jcm11133830. J Clin Med. 2022. PMID: 35807113 Free PMC article.
-
Role of Ezrin/Radixin/Moesin in the Surface Localization of Programmed Cell Death Ligand-1 in Human Colon Adenocarcinoma LS180 Cells.Pharmaceuticals (Basel). 2021 Aug 28;14(9):864. doi: 10.3390/ph14090864. Pharmaceuticals (Basel). 2021. PMID: 34577564 Free PMC article.
-
Subcellular distribution of ezrin/radixin/moesin and their roles in the cell surface localization and transport function of P-glycoprotein in human colon adenocarcinoma LS180 cells.PLoS One. 2021 May 11;16(5):e0250889. doi: 10.1371/journal.pone.0250889. eCollection 2021. PLoS One. 2021. PMID: 33974673 Free PMC article.
-
Pathophysiological Roles of Ezrin/Radixin/Moesin Proteins.Biol Pharm Bull. 2017;40(4):381-390. doi: 10.1248/bpb.b16-01011. Biol Pharm Bull. 2017. PMID: 28381792 Review.
-
Two Sides of the Coin: Ezrin/Radixin/Moesin and Merlin Control Membrane Structure and Contact Inhibition.Int J Mol Sci. 2019 Apr 23;20(8):1996. doi: 10.3390/ijms20081996. Int J Mol Sci. 2019. PMID: 31018575 Free PMC article. Review.
Cited by
-
Cellular Membrane Localization of Innate Immune Checkpoint Molecule CD47 Is Regulated by Radixin in Human Pancreatic Ductal Adenocarcinoma Cells.Biomedicines. 2023 Apr 7;11(4):1117. doi: 10.3390/biomedicines11041117. Biomedicines. 2023. PMID: 37189735 Free PMC article.
-
Ez-Metastasizing: The Crucial Roles of Ezrin in Metastasis.Cells. 2023 Jun 14;12(12):1620. doi: 10.3390/cells12121620. Cells. 2023. PMID: 37371090 Free PMC article. Review.
-
Uncharacterized Proteins CxORFx: Subinteractome Analysis and Prognostic Significance in Cancers.Int J Mol Sci. 2023 Jun 15;24(12):10190. doi: 10.3390/ijms241210190. Int J Mol Sci. 2023. PMID: 37373333 Free PMC article.
-
Ezrin Contributes to the Plasma Membrane Expression of PD-L1 in A2780 Cells.J Clin Med. 2022 Apr 27;11(9):2457. doi: 10.3390/jcm11092457. J Clin Med. 2022. PMID: 35566582 Free PMC article.
-
Moesin Serves as Scaffold Protein for PD-L1 in Human Uterine Cervical Squamous Carcinoma Cells.J Clin Med. 2022 Jul 1;11(13):3830. doi: 10.3390/jcm11133830. J Clin Med. 2022. PMID: 35807113 Free PMC article.

