Oclacitinib, a Janus Kinase Inhibitor, Reduces the Frequency of IL-4- and IL-10-, but Not IFN-γ-, Producing Murine CD4+ and CD8+ T Cells and Counteracts the Induction of Type 1 Regulatory T Cells
- PMID: 34577127
- PMCID: PMC8472008
- DOI: 10.3390/molecules26185655
Oclacitinib, a Janus Kinase Inhibitor, Reduces the Frequency of IL-4- and IL-10-, but Not IFN-γ-, Producing Murine CD4+ and CD8+ T Cells and Counteracts the Induction of Type 1 Regulatory T Cells
Abstract
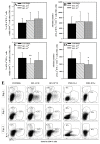
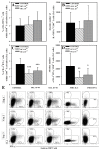
The purpose of the present study was to broaden the knowledge and understanding of the effects of oclacitinib (OCL), a Janus kinase inhibitor, on T cells in the context of both the immune mechanisms underlying anti-inflammatory and anti-allergic properties of the drug and its safety. The results indicate that beneficial effects of OCL in the treatment of skin allergic diseases may be partially mediated by the inhibition of IL-4 production in CD4+ and CD8+ T cells. To a certain extent, the antiproliferative effect of OCL on CD8+ T cells may also contribute to its therapeutic effect. The study found that OCL does not affect the proliferation of CD4+ T cells or the number of IFN-γ- and IL-17-producing CD4+ and CD8+ T cells. Moreover, OCL was found to counteract the induction of type 1 regulatory T (Tr1) cells and to act as a strong inhibitor of IL-10 production in both CD4+ and CD8+ T cells. Thus, these results indicate that beneficial effects of OCL in the treatment of skin allergic diseases are not mediated through: (a) the abolishment of IFN-γ and IL-17-production in CD4+ and CD8+ T cells; (b) generation of Tr1 cells; (c) inhibition of CD4+ T cell proliferation; (d) induction of IL-10 production in CD4+ T cells. The results of this study strongly suggest that, with respect to the evaluated parameters, OCL exerts a suppressive effect on Th2- but not Th1-mediated immunity.
Keywords: CD4+ cells; CD8+ cells; JAK; cytokines; oclacitinib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hodge J.A., Kawabata T.T., Krishnaswami S., Clark J.D., Telliez J.B., Dowty M.E., Menon S., Lamba M., Zwillich S. The mechanism of action of tofacitinib—An oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 2016;34:318–328. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

