Neurosteroids: Structure-Uptake Relationships and Computational Modeling of Organic Anion Transporting Polypeptides (OATP)1A2
- PMID: 34577133
- PMCID: PMC8472597
- DOI: 10.3390/molecules26185662
Neurosteroids: Structure-Uptake Relationships and Computational Modeling of Organic Anion Transporting Polypeptides (OATP)1A2
Abstract
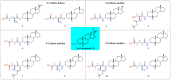
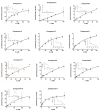
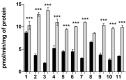
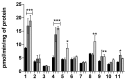
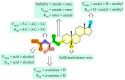
In this study, we investigated the delivery of synthetic neurosteroids into MCF-7 human breast adenocarcinoma cells via Organic Anionic Transporting Polypeptides (OATPs) (pH 7.4 and 5.5) to identify the structural components required for OATP-mediated cellular uptake and to get insight into brain drug delivery. Then, we identified structure-uptake relationships using in-house developed OATP1A2 homology model to predict binding sites and modes for the ligands. These binding modes were studied by molecular dynamics simulations to rationalize the experimental results. Our results show that carboxylic acid needs to be at least at 3 carbon-carbon bonds distance from amide bond at the C-3 position of the androstane skeleton and have an amino group to avoid efflux transport. Replacement of hydroxyl group at C-3 with any of the 3, 4, and 5-carbon chained terminal carboxylic groups improved the affinity. We attribute this to polar interactions between carboxylic acid and side-chains of Lys33 and Arg556. The additional amine group showed interactions with Glu172 and Glu200. Based on transporter capacities and efficacies, it could be speculated that the functionalization of acetyl group at the C-17 position of the steroidal skeleton might be explored further to enable OAT1A2-mediated delivery of neurosteroids into the cells and also across the blood-brain barrier.
Keywords: Organic Anion Transporting Polypeptides (OATPs); cellular uptake; docking; molecular modeling; neurosteroid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Organic anion transporting polypeptides of the OATP/SLCO superfamily: identification of new members in nonmammalian species, comparative modeling and a potential transport mode.J Membr Biol. 2005 Dec;208(3):213-27. doi: 10.1007/s00232-005-7004-x. Epub 2006 Apr 20. J Membr Biol. 2005. PMID: 16648940
-
The fluorescence-based competitive counterflow assay developed for organic anion transporting polypeptides 1A2, 1B1, 1B3 and 2B1 identifies pentamidine as a selective OATP1A2 substrate.FASEB J. 2023 Nov;37(11):e23223. doi: 10.1096/fj.202300530RR. FASEB J. 2023. PMID: 37781971
-
Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters.Am J Physiol Cell Physiol. 2009 Mar;296(3):C570-82. doi: 10.1152/ajpcell.00436.2008. Epub 2009 Jan 7. Am J Physiol Cell Physiol. 2009. PMID: 19129463
-
The role of organic anion transporting polypeptides in drug absorption, distribution, excretion and drug-drug interactions.Expert Opin Drug Metab Toxicol. 2017 Apr;13(4):409-424. doi: 10.1080/17425255.2017.1253679. Epub 2016 Nov 9. Expert Opin Drug Metab Toxicol. 2017. PMID: 27783531 Review.
-
Clinical significance of organic anion transporting polypeptides (OATPs) in drug disposition: their roles in hepatic clearance and intestinal absorption.Biopharm Drug Dispos. 2013 Jan;34(1):45-78. doi: 10.1002/bdd.1823. Biopharm Drug Dispos. 2013. PMID: 23115084 Review.
Cited by
-
Structural Dynamics of OATP1A2 in Mediating Paclitaxel Transport Mechanism in Breast Cancer.Nanotheranostics. 2025 Feb 3;9(1):52-62. doi: 10.7150/ntno.103095. eCollection 2025. Nanotheranostics. 2025. PMID: 40078312 Free PMC article.
-
Preparation, Characterization, and Evaluation of Liposomes Containing Oridonin from Rabdosia rubescens.Molecules. 2022 Jan 27;27(3):860. doi: 10.3390/molecules27030860. Molecules. 2022. PMID: 35164121 Free PMC article.
-
Targeting Glial Cells by Organic Anion-Transporting Polypeptide 1C1 (OATP1C1)-Utilizing l-Thyroxine-Derived Prodrugs.J Med Chem. 2023 Nov 23;66(22):15094-15114. doi: 10.1021/acs.jmedchem.3c01026. Epub 2023 Nov 6. J Med Chem. 2023. PMID: 37930268 Free PMC article.
-
Comparative Modelling of Organic Anion Transporting Polypeptides: Structural Insights and Comparison of Binding Modes.Molecules. 2022 Dec 3;27(23):8531. doi: 10.3390/molecules27238531. Molecules. 2022. PMID: 36500622 Free PMC article.
References
-
- Hua Y.-G., Han L.-P., Yang Q.-Q., Wang M.-J., Zhang E., Liu H.-M. A practical and efficient stereoselective synthesis of (S)-rivastigmine and (R)-rivastigmine. ChemistrySelect. 2018;3:1385–1387. doi: 10.1002/slct.201703032. - DOI
MeSH terms
Substances
Grants and funding
- 256837, 294227, 294229, 307057, 311939/Academy of Finland
- OP RDE (Project IOCB MSCA Mobility IV, No. CZ.02.2.69/0.0/0.0/20_079/0017783)/European Social Fund
- ERDF/ESF Project "PharmaBrain" No. CZ.02.1.01/0.0/0.0/16_025/0007444/European Regional Development Fund
- RVO 61388963/Academy of Sciences of the Czech Republic (AS CR)
LinkOut - more resources
Full Text Sources
Miscellaneous

