Synthesis of Biologically Relevant 1,2,3- and 1,3,4-Triazoles: From Classical Pathway to Green Chemistry
- PMID: 34577138
- PMCID: PMC8464795
- DOI: 10.3390/molecules26185667
Synthesis of Biologically Relevant 1,2,3- and 1,3,4-Triazoles: From Classical Pathway to Green Chemistry
Abstract
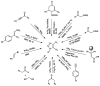
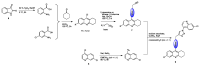
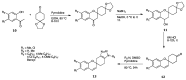
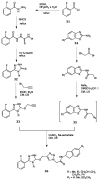
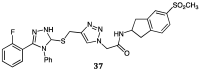
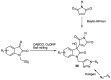
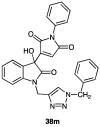
Green Chemistry has become in the last two decades an increasing part of research interest. Nonconventional «green» sources for chemical reactions include micro-wave, mechanical mixing, visible light and ultrasound. 1,2,3-triazoles have important applications in pharmaceutical chemistry while their 1,2,4 counterparts are developed to a lesser extent. In the review presented here we will focus on synthesis of 1,2,3 and 1,2,4-triazole systems by means of classical and « green chemistry » conditions involving ultrasound chemistry and mechanochemistry. The focus will be on compounds/scaffolds that possess biological/pharmacophoric properties. Finally, we will also present the formal cycloreversion of 1,2,3-triazole compounds under mechanical forces and its potential use in biological systems.
Keywords: 1,2,3-triazoles; 1,2,4-triazoles; biological properties; green chemistry; mechanochemistry; medicinal chemistry; ultrasound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
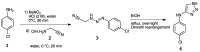

Similar articles
-
Green Approach Toward Triazole Forming Reactions for Developing Anticancer Drugs.Curr Org Synth. 2024;21(4):380-420. doi: 10.2174/1570179420666230508125144. Curr Org Synth. 2024. PMID: 37157212 Review.
-
Alternative Routes to the Click Method for the Synthesis of 1,2,3-Triazoles, an Important Heterocycle in Medicinal Chemistry.Curr Top Med Chem. 2018;18(17):1428-1453. doi: 10.2174/1568026618666180821143902. Curr Top Med Chem. 2018. PMID: 30129413 Review.
-
10 years of click chemistry: synthesis and applications of ferrocene-derived triazoles.Chem Asian J. 2011 Oct 4;6(10):2670-94. doi: 10.1002/asia.201100408. Epub 2011 Aug 31. Chem Asian J. 2011. PMID: 21882351 Review.
-
1,5-Disubstituted 1,2,3-triazoles: Molecular scaffolds for medicinal chemistry and biomolecular mimetics.Eur J Med Chem. 2025 Jul 5;291:117614. doi: 10.1016/j.ejmech.2025.117614. Epub 2025 Apr 10. Eur J Med Chem. 2025. PMID: 40239486 Review.
-
Green ultrasound-assisted three-component click synthesis of novel 1H-1,2,3-triazole carrying benzothiazoles and fluorinated-1,2,4-triazole conjugates and their antimicrobial evaluation.Acta Pharm. 2017 Sep 1;67(3):309-324. doi: 10.1515/acph-2017-0024. Acta Pharm. 2017. PMID: 28858836
Cited by
-
Quasi-Podands with 1,2,3-Triazole Rings from Bile Acid Derivatives: Synthesis, and Spectroscopic and Theoretical Studies.J Org Chem. 2024 Jun 7;89(11):7561-7572. doi: 10.1021/acs.joc.4c00195. Epub 2024 May 14. J Org Chem. 2024. PMID: 38743871 Free PMC article.
-
New triazole-based hybrids as neurotropic agents.RSC Adv. 2024 Oct 18;14(45):32922-32943. doi: 10.1039/d4ra06121g. eCollection 2024 Oct 17. RSC Adv. 2024. PMID: 39429923 Free PMC article.
-
New Bicyclic Pyridine-Based Hybrids Linked to the 1,2,3-Triazole Unit: Synthesis via Click Reaction and Evaluation of Neurotropic Activity and Molecular Docking.Molecules. 2023 Jan 17;28(3):921. doi: 10.3390/molecules28030921. Molecules. 2023. PMID: 36770592 Free PMC article.
-
Design, Synthesis, and Testing of 1,2,3-Triazolo-Quinobenzothiazine Hybrids for Cytotoxic and Immunomodulatory Activity.Int J Mol Sci. 2025 Jul 18;26(14):6920. doi: 10.3390/ijms26146920. Int J Mol Sci. 2025. PMID: 40725171 Free PMC article.
-
Green and recyclable catalyst based on chitosan/CuFe2O4 nanocomposite hydrogel for one-step synthesis of 1,2,3-triazoles.RSC Adv. 2024 Oct 1;14(43):31320-31331. doi: 10.1039/d4ra05626d. eCollection 2024 Oct 1. RSC Adv. 2024. PMID: 39359334 Free PMC article.
References
-
- Anastas P.T., Warner J.C. Green Chemistry, Theory and Practice. Oxford University Press; Oxford, UK, 2000:
-
- Poliakoff M., Licence P. Sustainable Technology: Green Chemistry. [(accessed on 16 September 2018)]. Available online: https://www.nature.com/articles/450810a.
-
- Varma R.S. Chemical Activation by Mechanochemical Mixing, Microwave and Ultrasonic Irradiation. Green Chem. 2008;10:1129–1130. doi: 10.1039/b817559b. - DOI
-
- Mason T.J., Lorimer J.P. Applied Sonochemistry: Uses of Power Ultrasound in Chemistry and Processing. Wiley-VCH Verlag GmbH & Co. KgaA; Weinheim, Germany: 2002.
-
- Bonrath W., Schmidt R.A.P. Ultrasound in Synthetic Organic Chemistry. Adv. Org. Synth. 2005;1:81–117. doi: 10.2174/1574087054582923. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

