Flaxseed Increases Animal Lifespan and Reduces Ovarian Cancer Severity by Toxically Augmenting One-Carbon Metabolism
- PMID: 34577143
- PMCID: PMC8471351
- DOI: 10.3390/molecules26185674
Flaxseed Increases Animal Lifespan and Reduces Ovarian Cancer Severity by Toxically Augmenting One-Carbon Metabolism
Abstract
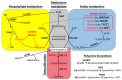
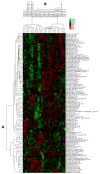
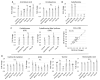
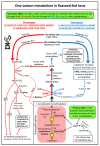
We used an LC-MS/MS metabolomics approach to investigate one-carbon metabolism in the plasma of flaxseed-fed White Leghorn laying hens (aged 3.5 years). In our study, dietary flaxseed (via the activity of a vitamin B6 antagonist known as "1-amino d-proline") induced at least 15-fold elevated plasma cystathionine. Surprisingly, plasma homocysteine (Hcy) was stable in flaxseed-fed hens despite such highly elevated cystathionine. To explain stable Hcy, our data suggest accelerated Hcy remethylation via BHMT and MS-B12. Also supporting accelerated Hcy remethylation, we observed elevated S-adenosylmethionine (SAM), an elevated SAM:SAH ratio, and elevated methylthioadenosine (MTA), in flaxseed-fed hens. These results suggest that flaxseed increases SAM biosynthesis and possibly increases polyamine biosynthesis. The following endpoint phenotypes were observed in hens consuming flaxseed: decreased physiological aging, increased empirical lifespan, 9-14% reduced body mass, and improved liver function. Overall, we suggest that flaxseed can protect women from ovarian tumor metastasis by decreasing omental adiposity. We also propose that flaxseed protects cancer patients from cancer-associated cachexia by enhancing liver function.
Keywords: aging; cancer; chicken; diet; flaxseed; lifespan; liver; metabolism; nutrition; ovarian.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Flaxseed enriched diet-mediated reduction in ovarian cancer severity is correlated to the reduction of prostaglandin E(2) in laying hen ovaries.Prostaglandins Leukot Essent Fatty Acids. 2013 Sep;89(4):179-87. doi: 10.1016/j.plefa.2013.08.001. Epub 2013 Aug 14. Prostaglandins Leukot Essent Fatty Acids. 2013. PMID: 23978451 Free PMC article. Clinical Trial.
-
Long term consumption of flaxseed enriched diet decreased ovarian cancer incidence and prostaglandin E₂in hens.Gynecol Oncol. 2013 Sep;130(3):620-8. doi: 10.1016/j.ygyno.2013.05.018. Epub 2013 May 23. Gynecol Oncol. 2013. PMID: 23707669 Free PMC article.
-
Decreased severity of ovarian cancer and increased survival in hens fed a flaxseed-enriched diet for 1 year.Gynecol Oncol. 2010 May;117(2):341-7. doi: 10.1016/j.ygyno.2010.01.021. Epub 2010 Feb 13. Gynecol Oncol. 2010. PMID: 20153884 Free PMC article.
-
Hyperhomocysteinemia and response of methionine cycle intermediates to vitamin treatment in renal patients.Clin Chem Lab Med. 2005;43(10):1039-47. doi: 10.1515/CCLM.2005.182. Clin Chem Lab Med. 2005. PMID: 16197295 Review.
-
The role of the mitochondrial trans-sulfuration in cerebro-cardio renal dysfunction during trisomy down syndrome.Mol Cell Biochem. 2024 Apr;479(4):825-829. doi: 10.1007/s11010-023-04761-9. Epub 2023 May 17. Mol Cell Biochem. 2024. PMID: 37198322 Review.
Cited by
-
Physiological and Pathological Considerations for the Use of Flaxseed as a Therapeutic Dietary Strategy.Rev Cardiovasc Med. 2023 May 18;24(5):149. doi: 10.31083/j.rcm2405149. eCollection 2023 May. Rev Cardiovasc Med. 2023. PMID: 39076734 Free PMC article. Review.
-
Flaxseed Supplementation in Chicken Feed Accelerates Salmonella enterica subsp. enterica Serovar Enteritidis Clearance, Modulates Cecum Microbiota, and Influences Ovarian Gene Expression in Laying Hens.Biomolecules. 2023 Sep 6;13(9):1353. doi: 10.3390/biom13091353. Biomolecules. 2023. PMID: 37759753 Free PMC article.
-
Flaxseed Reduces Cancer Risk by Altering Bioenergetic Pathways in Liver: Connecting SAM Biosynthesis to Cellular Energy.Metabolites. 2023 Aug 14;13(8):945. doi: 10.3390/metabo13080945. Metabolites. 2023. PMID: 37623888 Free PMC article.
-
On the Path towards a "Greener" EU: A Mini Review on Flax (Linum usitatissimum L.) as a Case Study.Plants (Basel). 2023 Mar 1;12(5):1102. doi: 10.3390/plants12051102. Plants (Basel). 2023. PMID: 36903961 Free PMC article. Review.
-
Phytoestrogen Signal Pathways and Estrogen Signaling in Ovarian Cancer: A Narrative Review.Phytother Res. 2025 Aug;39(8):3601-3612. doi: 10.1002/ptr.70013. Epub 2025 Jul 9. Phytother Res. 2025. PMID: 40634118 Free PMC article. Review.
References
-
- Barua A., Bitterman P., Abramowicz J.S., Dirks A.L., Bahr J.M., Hales D.B., Bradaric M.J., Edassery S.L., Rotmensch J., Luborsky J.L. Histopathology of ovarian tumors in laying hens, a preclinical model of human ovarian cancer. Int. J. Gynecol. Cancer. 2009;19:531–539. doi: 10.1111/IGC.0b013e3181a41613. - DOI - PMC - PubMed
-
- Hakim A.A., Barry C.P., Barnes H.J., Anderson K.E., Petitte J., Whitaker R., Lancaster J.M., Wenham R.M., Carver D.K., Turbov J., et al. Ovarian Adenocarcinomas in the Laying Hen and Women Share Similar Alterations in p53, ras, and HER-2/neu. Cancer Prev. Res. 2009;2:114–121. doi: 10.1158/1940-6207.CAPR-08-0065. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

