Novel c-Jun N-Terminal Kinase (JNK) Inhibitors with an 11 H-Indeno[1,2- b]quinoxalin-11-one Scaffold
- PMID: 34577159
- PMCID: PMC8464905
- DOI: 10.3390/molecules26185688
Novel c-Jun N-Terminal Kinase (JNK) Inhibitors with an 11 H-Indeno[1,2- b]quinoxalin-11-one Scaffold
Abstract
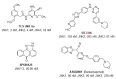
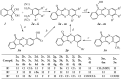
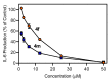
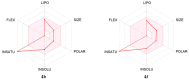
c-Jun N-terminal kinase (JNK) plays a central role in stress signaling pathways implicated in important pathological processes, including rheumatoid arthritis and ischemia-reperfusion injury. Therefore, inhibition of JNK is of interest for molecular targeted therapy to treat various diseases. We synthesized 13 derivatives of our reported JNK inhibitor 11H-indeno[1,2-b]quinoxalin-11-one oxime and evaluated their binding to the three JNK isoforms and their biological effects. Eight compounds exhibited submicromolar binding affinity for at least one JNK isoform. Most of these compounds also inhibited lipopolysaccharide (LPS)-induced nuclear factor-κB/activating protein 1 (NF-κB/AP-1) activation and interleukin-6 (IL-6) production in human monocytic THP1-Blue cells and human MonoMac-6 cells, respectively. Selected compounds (4f and 4m) also inhibited LPS-induced c-Jun phosphorylation in MonoMac-6 cells, directly confirming JNK inhibition. We conclude that indenoquinoxaline-based oximes can serve as specific small-molecule modulators for mechanistic studies of JNKs, as well as potential leads for the development of anti-inflammatory drugs.
Keywords: 11H-indeno[1,2-b]quinoxalin-11-one; c-Jun N-terminal kinase; interleukin-6; kinase inhibitor; nuclear factor-κB; oxime.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Synthesis, biological evaluation, and molecular modeling of 11H-indeno[1,2-b]quinoxalin-11-one derivatives and tryptanthrin-6-oxime as c-Jun N-terminal kinase inhibitors.Eur J Med Chem. 2019 Jan 1;161:179-191. doi: 10.1016/j.ejmech.2018.10.023. Epub 2018 Oct 12. Eur J Med Chem. 2019. PMID: 30347329 Free PMC article.
-
Identification and characterization of a novel class of c-Jun N-terminal kinase inhibitors.Mol Pharmacol. 2012 Jun;81(6):832-45. doi: 10.1124/mol.111.077446. Epub 2012 Mar 20. Mol Pharmacol. 2012. PMID: 22434859 Free PMC article.
-
Inhibitory effect of IQ-1S, a selective c-Jun N-terminal kinase (JNK) inhibitor, on phenotypical and cytokine-producing characteristics in human macrophages and T-cells.Eur J Pharmacol. 2020 Jul 5;878:173116. doi: 10.1016/j.ejphar.2020.173116. Epub 2020 Apr 18. Eur J Pharmacol. 2020. PMID: 32315671
-
Recent progress in the design, study, and development of c-Jun N-terminal kinase inhibitors as anticancer agents.Chem Biol. 2014 Nov 20;21(11):1433-43. doi: 10.1016/j.chembiol.2014.09.007. Epub 2014 Oct 16. Chem Biol. 2014. PMID: 25442375 Review.
-
JNK inhibitors as anti-inflammatory and neuroprotective agents.Future Med Chem. 2013 Apr;5(5):539-51. doi: 10.4155/fmc.13.34. Future Med Chem. 2013. PMID: 23573972 Review.
Cited by
-
Targeting transcription factors for therapeutic benefit in rheumatoid arthritis.Front Immunol. 2023 Jun 29;14:1196931. doi: 10.3389/fimmu.2023.1196931. eCollection 2023. Front Immunol. 2023. PMID: 37457726 Free PMC article. Review.
-
Novel Tryptanthrin Derivatives with Selectivity as c-Jun N-Terminal Kinase (JNK) 3 Inhibitors.Molecules. 2023 Jun 16;28(12):4806. doi: 10.3390/molecules28124806. Molecules. 2023. PMID: 37375361 Free PMC article.
-
Experimental and Computational Investigation of the Oxime Bond Stereochemistry in c-Jun N-terminal Kinase 3 Inhibitors 11H-Indeno[1,2-b]quinoxalin-11-one Oxime and Tryptanthrin-6-oxime.Pharmaceutics. 2023 Jun 23;15(7):1802. doi: 10.3390/pharmaceutics15071802. Pharmaceutics. 2023. PMID: 37513989 Free PMC article.
-
Design, synthesis and biological evaluation of novel O-substituted tryptanthrin oxime derivatives as c-Jun N-terminal kinase inhibitors.Front Pharmacol. 2022 Sep 12;13:958687. doi: 10.3389/fphar.2022.958687. eCollection 2022. Front Pharmacol. 2022. PMID: 36172181 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

