Electrophilically Activated Nitroalkanes in Double Annulation of [1,2,4]Triazolo[4,3- a]quinolines and 1,3,4-Oxadiazole Rings
- PMID: 34577163
- PMCID: PMC8464891
- DOI: 10.3390/molecules26185692
Electrophilically Activated Nitroalkanes in Double Annulation of [1,2,4]Triazolo[4,3- a]quinolines and 1,3,4-Oxadiazole Rings
Abstract
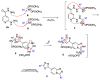
Nitroalkanes activated with polyphosphoric acid could serve as efficient electrophiles in reactions with amines and hydrazines, enabling various cascade transformations toward heterocyclic systems. This strategy was developed for an innovative synthetic protocol employing simultaneous or sequential annulation of two different heterocyclic cores, affording [1,2,4]triazolo[4,3-a]quinolines with 1,3,4-oxadiazole substituents.
Keywords: annulation; cascade transformations; heterocycles; nitroalkanes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Electrophilically Activated Nitroalkanes in Synthesis of 3,4-Dihydroquinozalines.Molecules. 2021 Jul 14;26(14):4274. doi: 10.3390/molecules26144274. Molecules. 2021. PMID: 34299549 Free PMC article.
-
Nitroalkanes as electrophiles: synthesis of triazole-fused heterocycles with neuroblastoma differentiation activity.Org Biomol Chem. 2020 Sep 14;18(34):6651-6664. doi: 10.1039/d0ob01007c. Epub 2020 Aug 19. Org Biomol Chem. 2020. PMID: 32813002 Free PMC article.
-
Synthesis of imidazo[1,5-a]pyridines via cyclocondensation of 2-(aminomethyl)pyridines with electrophilically activated nitroalkanes.Beilstein J Org Chem. 2020 Nov 26;16:2903-2910. doi: 10.3762/bjoc.16.239. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 33299488 Free PMC article.
-
Electrophilically Activated Nitroalkanes in Reactions With Carbon Based Nucleophiles.Front Chem. 2020 Feb 11;8:77. doi: 10.3389/fchem.2020.00077. eCollection 2020. Front Chem. 2020. PMID: 32117896 Free PMC article. Review.
-
Cyanoacetohydrazides in Synthesis of Heterocyclic Compounds.Top Curr Chem (Cham). 2018 Oct 11;376(6):40. doi: 10.1007/s41061-018-0218-z. Top Curr Chem (Cham). 2018. PMID: 30306359 Review.
References
-
- Hu Q., Yin L., Ali A., Cooke A.J., Bennett J., Ratcliffe P., Lo M.M.-C., Metzger E., Hoyt S., Hartmann R.W. Novel pyridyl pubstituted 4,5-dihydro-[1,2,4]triazolo[4,3-a]quinolines as potent and pelective pldosterone pynthase pnhibitors with pmproved in vitro metabolic stability. J. Med. Chem. 2015;58:2530–2537. doi: 10.1021/acs.jmedchem.5b00079. - DOI - PubMed
-
- Aksenov N.A., Aksenov A.V., Kirilov N.K., Arutiunov N.A., Aksenov D.A., Maslivetc V., Zhao Z., Du L., Rubin M., Kornienko A. Nitroalkanes as electrophiles: Synthesis of triazole-fused heterocycles with neuroblastoma differentiation activity. Org. Biomol. Chem. 2020;18:6651–6664. doi: 10.1039/D0OB01007C. - DOI - PMC - PubMed
-
- Reddy B.N., Reddy P.V.G., Reddy P.S., Reddy S.M., Reddy S.R.S., Pathak M. Synthesis of New 4,5-dihydro-1-methyl-[1,2,4]triazolo[4,3-a]quinolin-7-amine-derived ureas and their anticancer activity. Synth. Commun. 2015;45:831–837. doi: 10.1080/00397911.2014.989449. - DOI
-
- Chen J., Sun X.-Y., Chai K.-Y., Lee J.-S., Song M.-S., Quan Z.-S. Synthesis and anticonvulsant evaluation of 4-(4-alkoxylphenyl)-3-ethyl-4H-1,2,4-triazoles as open-chain analogues of 7-alkoxyl-4,5-dihydro[1,2,4]triazolo[4,3-a]quinolines. Bioorg. Med. Chem. 2007;15:6775–6781. doi: 10.1016/j.bmc.2007.08.004. - DOI - PubMed
-
- Cui X., Guan L., Li H., Piao H., Quan Z. Synthesis and anticonvulsant activity of 7-benzylamino-4,5-dihydro-[1,2,4]triazolo[4,3-a]quinolines. Prog. Nat. Sci. 2007;17:1104–1108.
Grants and funding
LinkOut - more resources
Full Text Sources

