Copper(II)-Mediated Iodination of 1-Nitroso-2-naphthol
- PMID: 34577180
- PMCID: PMC8465374
- DOI: 10.3390/molecules26185708
Copper(II)-Mediated Iodination of 1-Nitroso-2-naphthol
Abstract
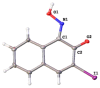
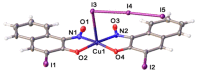
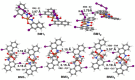
The 3-Iodo-1-nitrosonaphthalene-2-ol (I-NON) was obtained by the copper(II)-mediated iodination of 1-nitroso-2-naphthol (NON). The suitable reactants and optimized reaction conditions, providing 94% NMR yield of I-NON, included the usage of Cu(OAc)2·H2O and 1:2:8 CuII/NON/I2 molar ratio between the reactants. The obtained I-NON was characterized by elemental analyses (C, H, N), high-resolution ESI+-MS, 1H and 13C{1H} NMR, FTIR, UV-vis spectroscopy, TGA, and X-ray crystallography (XRD). The copper(II) complexes bearing deprotonated I-NON were prepared as follows: cis-[Cu(I-NON-H)(I-NON)](I3) (1) was obtained by the reaction between Cu(NON-H)2 and I2 in CHCl3/MeOH, while trans-[Cu(I-NON-H)2] (2) was synthesized from I-NON and Cu(OAc)2 in MeOH. Crystals of trans-[Cu(I-NON-H)2(THF)2] (3) and trans-[Cu(I-NON-H)2(Py)2] (4) were precipitated from solutions of 2 in CHCl3/THF and Py/CHCl3/MeOH mixtures, respectively. The structures of 1 and 3-4 were additionally verified by X-ray crystallography. The characteristic feature of the structures of 1 and 3 is the presence of intermolecular halogen bonds with the involvement of the iodine center of the metal-bound deprotonated I-NON. The nature of the I···I and I···O contacts in the structures of 1 and 3, correspondingly, were studied theoretically at the DFT (PBE0-D3BJ) level using the QTAIM, ESP, ELF, NBO, and IGM methods.
Keywords: copper(II) complexes; halogen bonding; iodination; metal-mediated reaction; nitrosonaphthol.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
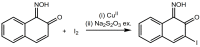




Similar articles
-
Copper and Bismuth Complexes Containing Dipyridyl gem-Diolato Ligands: Bi(III)(2)[(2-Py)(2)CO(OH)](2)(O(2)CCF(3))(4)(THF)(2), Cu(II)[(2-Py)(2)CO(OH)](2)(HO(2)CCH(3))(2), and Cu(II)(4)[(2-Py)(2)CO(OH)](2)(O(2)CCH(3))(6)(H(2)O)(2), a Ferromagnetically Coupled Tetranuclear Copper(II) Chain.Inorg Chem. 1996 Nov 20;35(24):6944-6951. doi: 10.1021/ic9602310. Inorg Chem. 1996. PMID: 11666871
-
Syntheses and characterization of copper(II) carboxylate dimers formed from enantiopure ligands containing a strong π···π stacking synthon: enantioselective single-crystal to single-crystal gas/solid-mediated transformations.Inorg Chem. 2011 Oct 17;50(20):10225-40. doi: 10.1021/ic201238n. Epub 2011 Sep 15. Inorg Chem. 2011. PMID: 21919476
-
Syntheses, characterization, and dioxygen reactivities of Cu(I) complexes with cis,cis-1,3,5-triaminocyclohexane derivatives: a Cu(III)2O2 intermediate exhibiting higher C-H activation.Inorg Chem. 2007 Apr 16;46(8):3322-35. doi: 10.1021/ic062206s. Epub 2007 Mar 20. Inorg Chem. 2007. PMID: 17371011
-
Steric and hydrogen-bonding effects on the stability of copper complexes with small molecules.Inorg Chem. 2004 Sep 6;43(18):5725-35. doi: 10.1021/ic0496572. Inorg Chem. 2004. PMID: 15332825
-
Dipeptides containing the alpha-aminoisobutyric residue (Aib) as ligands: preparation, spectroscopic studies and crystal structures of copper(II) complexes with H-Aib-X-OH (X=Gly, L-Leu, L-Phe).J Inorg Biochem. 2003 Jan 15;93(3-4):109-18. doi: 10.1016/s0162-0134(02)00560-3. J Inorg Biochem. 2003. PMID: 12576272
References
-
- Pike S.J., Heliot A., Seaton C.C. ortho-Substituent effect on the crystal packing and solid state speciation of aromatic C-nitroso compounds. CrystEngComm. 2020;22:5040–5048. doi: 10.1039/D0CE00728E. - DOI
-
- Raić-Malić S., Tomašković L., Mrvoš-Sermek D., Prugovečki B., Cetina M., Grdiša M., Pavelić K., Mannschreck A., Balzarini J., De Clercq E., et al. Spirobipyridopyrans, spirobinaphthopyrans, indolinospiropyridopyrans, indolinospironaphthopyrans and indolinospironaphtho-1,4-oxazines: Synthesis, study of X-ray crystal structure, antitumoral and antiviral evaluation. Bioorg. Med. Chem. 2004;12:1037–1045. doi: 10.1016/j.bmc.2003.12.010. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

