Preparation of Solid Dispersions of Simvastatin and Soluplus Using a Single-Step Organic Solvent-Free Supercritical Fluid Process for the Drug Solubility and Dissolution Rate Enhancement
- PMID: 34577546
- PMCID: PMC8468910
- DOI: 10.3390/ph14090846
Preparation of Solid Dispersions of Simvastatin and Soluplus Using a Single-Step Organic Solvent-Free Supercritical Fluid Process for the Drug Solubility and Dissolution Rate Enhancement
Abstract
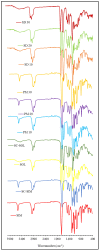
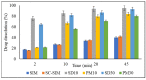
The study was designed to investigate the feasibility of supercritical carbon dioxide (scCO2) processing for the preparation of simvastatin (SIM) solid dispersions (SDs) in Soluplus® (SOL) at temperatures below polymer's glass transition. The SIM content in the SDs experimental design was kept at 10, 20 and 30% to study the effect of the drug-polymer ratio on the successful preparation of SDs. The SIM-SOL formulations, physical mixtures (PMs) and SDs were evaluated using X-ray diffraction (XRD), differential scanning calorimetry (DSC), attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), scanning electron microscopy (SEM), and dissolution studies. The scCO2 processing conditions and drug-polymer ratio were found to influence the physicochemical properties of the drug in formulated SDs. SIM is a highly crystalline drug; however, physicochemical characterisation carried out by SEM, DSC, and XRD demonstrated the presence of SIM in amorphous nature within the SDs. The SIM-SOL SDs showed enhanced drug dissolution rates, with 100% being released within 45 min. Moreover, the drug dissolution from SDs was faster and higher in comparison to PMs. In conclusion, this study shows that SIM-SOL dispersions can be successfully prepared using a solvent-free supercritical fluid process to enhance dissolution rate of the drug.
Keywords: drug dissolution; simvastatin; soluplus; supercritical carbon dioxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Singh D., Bedi N., Tiwary A.K. Enhancing solubility of poorly aqueous soluble drugs: Critical appraisal of techniques. J. Pharm. Investig. 2018;48:509–526. doi: 10.1007/s40005-017-0357-1. - DOI
-
- Dhirendra K., Lewis S., Udupa N., Atin K. Solid dispersions: A review. Pak. J. Pharm. Sci. 2009;22:234–246. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

