Development and Validation of a New Storage Procedure to Extend the In-Use Stability of Azacitidine in Pharmaceutical Formulations
- PMID: 34577643
- PMCID: PMC8470010
- DOI: 10.3390/ph14090943
Development and Validation of a New Storage Procedure to Extend the In-Use Stability of Azacitidine in Pharmaceutical Formulations
Abstract
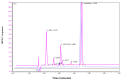
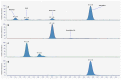
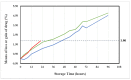
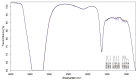
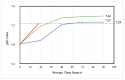
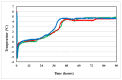
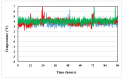
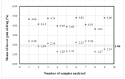
Stability studies performed by the pharmaceutical industry are principally designed to fulfill licensing requirements. Thus, post-dilution or post-reconstitution stability data are frequently limited to 24 h only for bacteriological reasons, regardless of the true physicochemical stability which could, in many cases, be longer. In practice, the pharmacy-based centralized preparation may require preparation in advance for administration, for example, on weekends, holidays, or in general when pharmacies may be closed. We report an innovative strategy for storing resuspended solutions of azacitidine, a well-known chemotherapic agent, for which the manufacturer lists maximum stability of 22 h. By placing the syringe with the azacitidine reconstituted suspension between two refrigerant gel packs and storing it at 4 °C, we found that the concentration of azacitidine remained above 98% of the initial concentration for 48 h, and no change in color nor the physicochemical properties of the suspension were observed throughout the study period. The physicochemical and microbiological properties were evaluated by HPLC-UV and UHPLC-HRMS analysis, FTIR spectroscopy, pH determination, visual and subvisual examination, and sterility assay. The HPLC-UV method used for evaluating the chemical stability of azacitidine was validated according to ICH. Precise control of storage temperature was obtained by a digital data logger. Our study indicates that by changing the storage procedure of azacitidine reconstituted suspension, the usage window of the drug can be significantly extended to a time frame that better copes with its use in the clinical environment.
Keywords: anticancer drugs; azacitidine; drug degradation; in-use stability; limits of use; practical stability.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
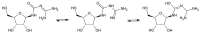




References
-
- Bardin C., Astier A., Vulto A., Sewell G., Vigneron J., Trittler R., Daouphars M., Paul M., Trojniak M., Pinguet F. Guidelines for the practical stability studies of anticancer drugs: A European consensus conference. Recommandations pour les essais de stabilité pratique des médicaments anticancéreux: Une conférence de consensus européenne. Ann. Pharm. Françaises. 2011;69:221–231. doi: 10.1016/j.pharma.2011.07.002. - DOI - PubMed
-
- European Medicines Agency. Committee for Proprietary Medicinal Products (CPMP) Note for Guidance in In-Use Stability Testing of Human Medicinal Product. The European Agency for the Evaluation of Medicinal Products; London, UK: 2001.
-
- European Medicines Agency Summary of Product Characteristics: Azacitidine. [(accessed on 5 May 2021)];2013 Available online: https://www.ema.europa.eu/en/documents/product-information/vidaza-epar-p....
LinkOut - more resources
Full Text Sources

