Microfluidic Device for Droplet Pairing by Combining Droplet Railing and Floating Trap Arrays
- PMID: 34577720
- PMCID: PMC8470175
- DOI: 10.3390/mi12091076
Microfluidic Device for Droplet Pairing by Combining Droplet Railing and Floating Trap Arrays
Abstract
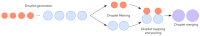
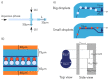
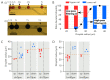
Droplet microfluidics are characterized by the generation and manipulation of discrete volumes of solutions, generated with the use of immiscible phases. Those droplets can then be controlled, transported, analyzed or their content modified. In this wide droplet microfluidic toolbox, no means are available to generate, in a controlled manner, droplets co-encapsulating to aqueous phases. Indeed, current methods rely on random co-encapsulation of two aqueous phases during droplet generation or the merging of two random droplets containing different aqueous phases. In this study, we present a novel droplet microfluidic device to reliably and efficiently co-encapsulate two different aqueous phases in micro-droplets. In order to achieve this, we combined existing droplet microfluidic modules in a novel way. The different aqueous phases are individually encapsulated in droplets of different sizes. Those droplet populations are then filtered in order to position each droplet type towards its adequate trapping compartment in traps of a floating trap array. Single droplets, each containing a different aqueous phase, are thus paired and then merged. This pairing at high efficiency is achieved thanks to a unique combination of floating trap arrays, a droplet railing system and a droplet size-based filtering mechanism. The microfluidic chip design presented here provides a filtering threshold with droplets larger than 35 μm (big droplets) being deviated to the lower rail while droplets smaller than 20 μm (small droplets) remain on the upper rail. The effects of the rail height and the distance between the two (upper and lower) rails were investigated. The optimal trap dimensions provide a trapping efficiency of 100% for small and big droplets with a limited double trapping (both compartments of the traps filled with the same droplet type) of 5%. The use of electrocoalescence enables the generation of a droplet while co-encapsulating two aqueous phases. Using the presented microfluidic device libraries of 300 droplets, dual aqueous content can be generated in less than 30 min.
Keywords: droplet; microfluidics; pairing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Agresti J.J., Antipov E., Abate A.R., Ahn K., Rowat A.C., Baret J.C., Marquez M., Klibanov A.M., Griffiths A.D., Weitz D.A. Ultrahigh-throughput screening in drop-based microfluidics for directed evolution. Proc. Natl. Acad. Sci. USA. 2010;107:4004–4009. doi: 10.1073/pnas.0910781107. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

