The Effect of Dialkyl Peroxide Crosslinking on the Properties of LLDPE and UHMWPE
- PMID: 34577963
- PMCID: PMC8470150
- DOI: 10.3390/polym13183062
The Effect of Dialkyl Peroxide Crosslinking on the Properties of LLDPE and UHMWPE
Abstract
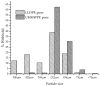
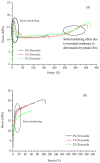
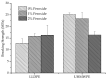
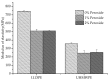
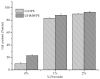
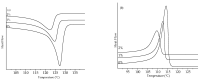
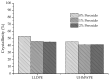
Peroxide has been considered a chemical agent that can be used to tune the properties of polymeric materials. This research evaluated the influence of different concentrations of dialkyl peroxides on the mechanical, thermal, and morphological properties of linear low-density polyethylene (LLDPE) and ultra-high molecular weight polyethylene (UHMWPE). The neat polymer, as well as those with the addition of 1% and 2% by mass of dialkyl peroxides, were subjected to compression molding and immersion in water for 1 h, under controlled temperatures of 90 °C. The values of the gel content found in the samples indicated that the addition of peroxide to the LLDPE and to the UHMWPE promoted the formation of a reticulated network. The structure obtained by the crosslinking led to less reorganization of the chains during the crystallization process, resulting in the formation of imperfect crystals and, consequently, in the reduction in melting temperatures, crystallization and enthalpy. The mechanical properties were altered with the presence of the crosslinker. The polymers presented had predominant characteristics of a ductile material, with the occurrence of crazing with an increased peroxide content.
Keywords: LLDPE; UHMWPE; crosslinking; peroxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Development and Characterization of LLDPE Blends with Different UHMWPE Concentrations Obtained by Hot Pressing.Polymers (Basel). 2022 Sep 6;14(18):3723. doi: 10.3390/polym14183723. Polymers (Basel). 2022. PMID: 36145867 Free PMC article.
-
Thermal and Mechanical Analysis of Polyethylene Homo-Composites Processed by Rotational Molding.Polymers (Basel). 2019 Mar 20;11(3):528. doi: 10.3390/polym11030528. Polymers (Basel). 2019. PMID: 30960512 Free PMC article.
-
Impact of Buriti Oil from Mauritia flexuosa Palm Tree on the Rheological, Thermal, and Mechanical Properties of Linear Low-Density Polyethylene for Improved Sustainability.Polymers (Basel). 2024 Oct 29;16(21):3037. doi: 10.3390/polym16213037. Polymers (Basel). 2024. PMID: 39518245 Free PMC article.
-
Polymer Physics behind the Gel-Spinning of UHMWPE Fibers.Macromol Rapid Commun. 2024 Aug;45(15):e2400124. doi: 10.1002/marc.202400124. Epub 2024 May 6. Macromol Rapid Commun. 2024. PMID: 38602184 Review.
-
Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty.Biomaterials. 1999 Sep;20(18):1659-88. doi: 10.1016/s0142-9612(99)00053-8. Biomaterials. 1999. PMID: 10503968 Review.
Cited by
-
Development and Characterization of LLDPE Blends with Different UHMWPE Concentrations Obtained by Hot Pressing.Polymers (Basel). 2022 Sep 6;14(18):3723. doi: 10.3390/polym14183723. Polymers (Basel). 2022. PMID: 36145867 Free PMC article.
-
Changes in Heat Resistance and Mechanical Properties of Peroxide Cross-Linking HDPE: Effects of Compounding Cross-Linkers.Polymers (Basel). 2025 Feb 19;17(4):535. doi: 10.3390/polym17040535. Polymers (Basel). 2025. PMID: 40006197 Free PMC article.
References
-
- Morshedian J., Hoseinpour P.M. Polyethylene Cross-linking by Two-step Silane Method: A Review. Iran. Polym. J. 2009;18:104–124.
-
- Zhao Y., Han Z., Xie Y., Fan X., Nie Y., Wang P., Liu G., Hao Y., Huang J., Zhu W. Correlation Between Thermal Parameters and Morphology of Cross-Linked Polyethylene. IEEE Access. 2020;8:19726–19736. doi: 10.1109/ACCESS.2020.2968109. - DOI
-
- Peacock A.J. Handbook of Polyethylene: Structures, Properties, and Applications. Marcel Dekker; New York, NY, USA: 2000. Plastics Engineering 57.
-
- Akiba M., Hashim A.S. Vulcanization and Crosslinking in Elastomers. Prog. Polym. Sci. 1997;22:457–521. doi: 10.1016/S0079-6700(96)00015-9. - DOI
-
- Kim K.J., Ok Y.S., Kim B.K. Crosslinking of Polyethylene with Peroxide and Multifunctional Monomers during Extrusion. Eur. Polym. J. 1992;28:1487–1491. doi: 10.1016/0014-3057(92)90140-W. - DOI
LinkOut - more resources
Full Text Sources

