Architecture and Composition Dictate Viscoelastic Properties of Organ-Derived Extracellular Matrix Hydrogels
- PMID: 34578013
- PMCID: PMC8470996
- DOI: 10.3390/polym13183113
Architecture and Composition Dictate Viscoelastic Properties of Organ-Derived Extracellular Matrix Hydrogels
Abstract
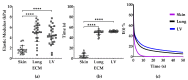
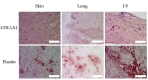
The proteins and polysaccharides of the extracellular matrix (ECM) provide architectural support as well as biochemical and biophysical instruction to cells. Decellularized, ECM hydrogels replicate in vivo functions. The ECM's elasticity and water retention renders it viscoelastic. In this study, we compared the viscoelastic properties of ECM hydrogels derived from the skin, lung and (cardiac) left ventricle and mathematically modelled these data with a generalized Maxwell model. ECM hydrogels from the skin, lung and cardiac left ventricle (LV) were subjected to a stress relaxation test under uniaxial low-load compression at a 20%/s strain rate and the viscoelasticity determined. Stress relaxation data were modelled according to Maxwell. Physical data were compared with protein and sulfated GAGs composition and ultrastructure SEM. We show that the skin-ECM relaxed faster and had a lower elastic modulus than the lung-ECM and the LV-ECM. The skin-ECM had two Maxwell elements, the lung-ECM and the LV-ECM had three. The skin-ECM had a higher number of sulfated GAGs, and a highly porous surface, while both the LV-ECM and the lung-ECM had homogenous surfaces with localized porous regions. Our results show that the elasticity of ECM hydrogels, but also their viscoelastic relaxation and gelling behavior, was organ dependent. Part of these physical features correlated with their biochemical composition and ultrastructure.
Keywords: ECM hydrogel; Maxwell model; decellularized organs; extracellular matrix; viscoelasticity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







Similar articles
-
Human lung extracellular matrix hydrogels resemble the stiffness and viscoelasticity of native lung tissue.Am J Physiol Lung Cell Mol Physiol. 2020 Apr 1;318(4):L698-L704. doi: 10.1152/ajplung.00451.2019. Epub 2020 Feb 12. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32048864 Free PMC article.
-
An in vitro model of fibrosis using crosslinked native extracellular matrix-derived hydrogels to modulate biomechanics without changing composition.Acta Biomater. 2022 Jul 15;147:50-62. doi: 10.1016/j.actbio.2022.05.031. Epub 2022 May 21. Acta Biomater. 2022. PMID: 35605955
-
Viscoelastic properties of plasma-agarose hydrogels dictate favorable fibroblast responses for skin tissue engineering applications.Biomater Adv. 2022 Aug;139:212967. doi: 10.1016/j.bioadv.2022.212967. Epub 2022 Jun 7. Biomater Adv. 2022. PMID: 35882126
-
Viscoelastic hydrogels for 3D cell culture.Biomater Sci. 2017 Jul 25;5(8):1480-1490. doi: 10.1039/c7bm00261k. Biomater Sci. 2017. PMID: 28584885 Review.
-
Extracellular matrix hydrogels from decellularized tissues: Structure and function.Acta Biomater. 2017 Feb;49:1-15. doi: 10.1016/j.actbio.2016.11.068. Epub 2016 Dec 1. Acta Biomater. 2017. PMID: 27915024 Free PMC article. Review.
Cited by
-
Fibroblasts alter the physical properties of dermal ECM-derived hydrogels to create a pro-angiogenic microenvironment.Mater Today Bio. 2023 Oct 24;23:100842. doi: 10.1016/j.mtbio.2023.100842. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 37942422 Free PMC article.
-
Matrix Metalloproteases from Adipose Tissue-Derived Stromal Cells Are Spatiotemporally Regulated by Hydrogel Mechanics in a 3D Microenvironment.Bioengineering (Basel). 2022 Jul 26;9(8):340. doi: 10.3390/bioengineering9080340. Bioengineering (Basel). 2022. PMID: 35892753 Free PMC article.
-
Innovative hydrogels in cutaneous wound healing: current status and future perspectives.Front Bioeng Biotechnol. 2025 May 12;13:1454903. doi: 10.3389/fbioe.2025.1454903. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40421113 Free PMC article. Review.
-
Adipose Tissue-Derived Components: From Cells to Tissue Glue to Treat Dermal Damage.Bioengineering (Basel). 2023 Mar 5;10(3):328. doi: 10.3390/bioengineering10030328. Bioengineering (Basel). 2023. PMID: 36978719 Free PMC article. Review.
-
Pulmonary matrix-derived hydrogels from patients with idiopathic pulmonary fibrosis induce a proinflammatory state in lung fibroblasts in vitro.Mol Biol Cell. 2024 Aug 1;35(8):ar114. doi: 10.1091/mbc.E23-11-0428. Epub 2024 Jul 10. Mol Biol Cell. 2024. PMID: 38985514 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

