Copper Rich Composite Materials Based on Carboxylic Cation Exchangers and Their Thermal Transformation
- PMID: 34578100
- PMCID: PMC8469408
- DOI: 10.3390/polym13183199
Copper Rich Composite Materials Based on Carboxylic Cation Exchangers and Their Thermal Transformation
Abstract
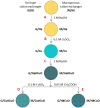
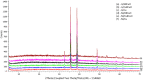
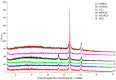
The effect of a cupric deposit (Cu2+, CuO) on the thermal decomposition of carboxylic cation exchangers (CCEs) is not known, and such studies may have practical significance. CCEs have a very high ion exchange capacity, so an exceptionally large amount of CuO (which is a catalyst) can be precipitated inside them. Two CCEs, macroreticular (Amberlite IRC50) and gel-like (Amberlite IRC86), served as a polymeric support to obtain copper-rich hybrid ion exchangers. Composites with CuO particles inside a polyacrylic matrix (up to 35.0 wt% Cu) were obtained. Thermal analyses under air and under N2 were performed for CCEs in the H+ and Cu2+ form with and without a CuO deposit. The results of sixteen experiments are discussed based on the TG/DTG curves and XRD patterns of the solid residues. Under air, the cupric deposit shifted the particular transformations and the ultimate polymeric matter decomposition (combustion) toward lower temperatures (even about 100-150 °C). Under N2, the reduction of the cupric deposit to metallic copper took place. Unique composite materials enriched in carbonaceous matter were obtained, as the products of polymeric matrix decomposition (free radicals and hydrogen) created an additional amount of carbon char due to the utilization of a certain amount of hydrogen to reduce Cu (II) to Cu0.
Keywords: carboxylic cation exchanger; composite; cuprous oxide; incineration; metallic copper; pyrolysis; thermal analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Hao J., Meng X., Fang S., Cao H., Jv W., Zheng X., Liu C., Chen M., Sun Z. MnO2 functionalized amorphous carbon sorbents from spent lithium-ion batteries for highly efficient removal of cadmium from aqueous solutions. Ind. Eng. Chem. Res. 2020;59:10210–10220. doi: 10.1021/acs.iecr.9b06670. - DOI
-
- Li R., Wang J.J., Gaston L.A., Zhou B., Li M., Xiao R., Wang Q., Zhang Z., Hui H., Liang W., et al. An overview of carbothermal synthesis of metal-biochar composites for the removal of oxyanion contaminants from aqueous solution. Carbon. 2018;129:674–687. doi: 10.1016/j.carbon.2017.12.070. - DOI
-
- Ghosh B.K., Hazra S., Naik B., Nath Ghosh N.N. Preparation of Cu nanoparticle loaded SBA-15 and their excellent catalytic activity in reduction of variety of dyes. Powder Technol. 2015;269:371–378. doi: 10.1016/j.powtec.2014.09.027. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

