The Liver and the Hepatic Immune Response in Trypanosoma cruzi Infection, a Historical and Updated View
- PMID: 34578107
- PMCID: PMC8465576
- DOI: 10.3390/pathogens10091074
The Liver and the Hepatic Immune Response in Trypanosoma cruzi Infection, a Historical and Updated View
Abstract
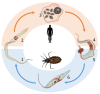
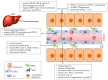
Chagas disease was described more than a century ago and, despite great efforts to understand the underlying mechanisms that lead to cardiac and digestive manifestations in chronic patients, much remains to be clarified. The disease is found beyond Latin America, including Japan, the USA, France, Spain, and Australia, and is caused by the protozoan Trypanosoma cruzi. Dr. Carlos Chagas described Chagas disease in 1909 in Brazil, and hepatomegaly was among the clinical signs observed. Currently, hepatomegaly is cited in most papers published which either study acutely infected patients or experimental models, and we know that the parasite can infect multiple cell types in the liver, especially Kupffer cells and dendritic cells. Moreover, liver damage is more pronounced in cases of oral infection, which is mainly found in the Amazon region. However, the importance of liver involvement, including the hepatic immune response, in disease progression does not receive much attention. In this review, we present the very first paper published approaching the liver's participation in the infection, as well as subsequent papers published in the last century, up to and including our recently published results. We propose that, after infection, activated peripheral T lymphocytes reach the liver and induce a shift to a pro-inflammatory ambient environment. Thus, there is an immunological integration and cooperation between peripheral and hepatic immunity, contributing to disease control.
Keywords: Chagas disease; Trypanosoma cruzi infection; hepatic immune response; liver.
Conflict of interest statement
The authors declare no conflict of interest of any nature.
Figures

References
-
- Chagas C. Nova especie morbida do homem, produzida por um trypanozoma (Trypanozoma cruzi) [(accessed on 22 March 2021)];Braz. Med. 1909 16 Available online: http://www.bvschagas.coc.fiocruz.br/lildbi/docsonline/get.php?id=527.
-
- Chagas C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. [(accessed on 22 March 2021)];Mem. Inst. Oswaldo Cruz. 1909 1:159–228. doi: 10.1590/S0074-02761909000200008. Available online: https://archive.org/details/memriasdoinsti12inst/page/n195/mode/2up. - DOI
-
- Chagas C. Ueber eine neue trypanosomiasis des menschen. Arch. Fiir Schiffs Trop. 1909;13:120–122.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

