Indian Herb-Derived Phytoconstituent-Based Antiviral, Antimicrobial and Antifungal Formulation: An Oral Rinse Candidate for Oral Hygiene and the Potential Prevention of COVID-19 Outbreaks
- PMID: 34578161
- PMCID: PMC8467774
- DOI: 10.3390/pathogens10091130
Indian Herb-Derived Phytoconstituent-Based Antiviral, Antimicrobial and Antifungal Formulation: An Oral Rinse Candidate for Oral Hygiene and the Potential Prevention of COVID-19 Outbreaks
Abstract
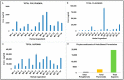
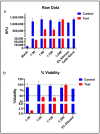
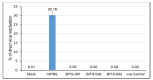
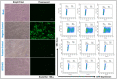
Outbreaks of emerging infectious diseases continue to challenge human health. Novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has triggered a global coronavirus pandemic, known as COVID-19. Multiple variants of SARS-CoV-2 virus are circulating, thus raising questions with respect to the effectiveness of different lines of treatment, such as vaccines and antiviral drugs. To find the appropriate prevention/treatment, 21 plant-based ingredients (Glycyrrhizin, Withanone, Aloe-emodin, Rhein, Emodin, Chrysophanol, Physcion, Kaempferol, Progallin A, Gallic acid, Naringin, Quercetin, Luteolin, and Apigenin) having antiviral, antibacterial and antifungal properties were identified. We pseudo-typed SARS-CoV-2 on a lentiviral vector plasmid and tested the impact of five different herbal formulations in mammalian HEK293T cells. Viral inactivation assay showed that the natural extracts in a herb-derived phytoconstituent-based formulation, BITS-003, comprising Bacopa monnieri, Glycyerrhiza glabra, Asparagus racemosus-wild, and Nigella sativa had strong virucidal properties, inactivating enveloped viruses from 2log10 (or 99%) to >4log10 (or 99.99%). Moreover, bacterial and yeast cells treated with BITS-003 displayed reduced growth. Topical use of the formulation as a mouthwash/gargle could be effective in reducing symptoms of respiratory viral infections, with the potential to decrease the viral load in the buccal/oral cavity. This may inhibit the coronavirus spreading to the lungs of infected persons and at the same time may reduce the risk of viral transmission to other susceptible persons through micro-droplets originating from the oral cavity of the infected person.
Keywords: COVID-19; SARS-CoV-2; antiviral agents; coronavirus; gargle; horizontal transmission; mouthwash; natural herb; phytoconstituents; shedding.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Natural Derived Nasal Spray; A Proposed Approach for COVID-19 Disease Control.Infect Disord Drug Targets. 2021;21(8):e160921191568. doi: 10.2174/1871526521666210218201113. Infect Disord Drug Targets. 2021. PMID: 33602078
-
Mechanistic Aspects of Medicinal Plants and Secondary Metabolites against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).Curr Pharm Des. 2021;27(38):3996-4007. doi: 10.2174/1381612827666210705160130. Curr Pharm Des. 2021. PMID: 34225607 Review.
-
Can povidone iodine gargle/mouthrinse inactivate SARS-CoV-2 and decrease the risk of nosocomial and community transmission during the COVID-19 pandemic? An evidence-based update.Jpn Dent Sci Rev. 2021 Nov;57:39-45. doi: 10.1016/j.jdsr.2021.03.001. Epub 2021 Mar 15. Jpn Dent Sci Rev. 2021. PMID: 33747261 Free PMC article. Review.
-
SARS-CoV-2 in environmental perspective: Occurrence, persistence, surveillance, inactivation and challenges.Chem Eng J. 2021 Feb 1;405:126893. doi: 10.1016/j.cej.2020.126893. Epub 2020 Sep 4. Chem Eng J. 2021. PMID: 32901196 Free PMC article.
-
Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2).Ear Nose Throat J. 2021 Apr;100(2_suppl):192S-196S. doi: 10.1177/0145561320957237. Epub 2020 Sep 21. Ear Nose Throat J. 2021. PMID: 32951446
Cited by
-
In vivo efficacy of 2% povidone iodine, chlorhexidine gluconate, and herbal extract mouthwash on SARS-CoV-2 viral load in saliva: A randomized clinical trial.J Indian Soc Periodontol. 2023 Nov-Dec;27(6):607-611. doi: 10.4103/jisp.jisp_469_22. Epub 2024 Jan 24. J Indian Soc Periodontol. 2023. PMID: 38434502 Free PMC article.
-
Asarum sieboldii, a Potential Ethnomedicinal Herb in Dentistry and Oral Health.Int Dent J. 2025 Aug;75(4):100816. doi: 10.1016/j.identj.2025.03.025. Epub 2025 May 5. Int Dent J. 2025. PMID: 40328202 Free PMC article. Review.
-
Efficacy of Mouth Rinses and Nasal Spray in the Inactivation of SARS-CoV-2: A Systematic Review and Meta-Analysis of In Vitro and In Vivo Studies.Int J Environ Res Public Health. 2022 Sep 25;19(19):12148. doi: 10.3390/ijerph191912148. Int J Environ Res Public Health. 2022. PMID: 36231450 Free PMC article.
-
Mitochondrial resilience and antioxidant defence against HIV-1: unveiling the power of Asparagus racemosus extracts and Shatavarin IV.Front Microbiol. 2024 Oct 23;15:1475457. doi: 10.3389/fmicb.2024.1475457. eCollection 2024. Front Microbiol. 2024. PMID: 39507335 Free PMC article.
-
Efficacy of a mouthwash containing ε-poly-L-lysine, funme peptides and domiphen in reducing halitosis and supragingival plaque: a randomized clinical trial.BMC Oral Health. 2024 May 3;24(1):525. doi: 10.1186/s12903-024-04255-0. BMC Oral Health. 2024. PMID: 38702623 Free PMC article. Clinical Trial.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

