An Exaggerated Immune Response in Female BALB/c Mice Controls Initial Toxoplasma gondii Multiplication but Increases Mortality and Morbidity Relative to Male Mice
- PMID: 34578186
- PMCID: PMC8470933
- DOI: 10.3390/pathogens10091154
An Exaggerated Immune Response in Female BALB/c Mice Controls Initial Toxoplasma gondii Multiplication but Increases Mortality and Morbidity Relative to Male Mice
Abstract
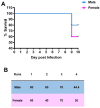
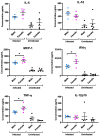
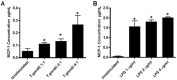
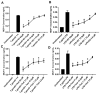
Studies indicate that female mice are more susceptible to T. gondii infection, as defined by higher mortality rates in comparison to male mice. However, whether this is due to an inability to control initial parasite multiplication or due to detrimental effects of the immune system has not been determined. Therefore, the following studies were undertaken to determine the influence of sex on early parasite multiplication and the immune response during T. gondii infection and to correlate this with disease outcome. Early parasite replication was studied through applying an in vivo imaging system (IVIS) with luciferase expressing T. gondii. In parallel immunological events were studied by cytometric bead array to quantify key immunological mediators. The results confirmed the previous findings that female mice are more susceptible to acute infection, as determined by higher mortality rates and weight loss compared with males. However, conflicting with expectations, female mice had lower parasite burdens during the acute infection than male mice. Female mice also exhibited significantly increased production of Monocyte Chemoattractant Protein-1 (MCP-1), Interferon (IFN)-γ, and Tumour Necrosis Factor (TNF)-α than male mice. MCP-1 was found to be induced by T. gondii in a dose dependent manner suggesting that the observed increased levels detected in female mice was due to a host-mediated sex difference rather than due to parasite load. However, MCP-1 was not affected by physiological concentration of estrogen or testosterone, indicating that MCP-1 differences observed between the sexes in vivo are due to an as yet unidentified intermediary factor that in turn influences MCP-1 levels. These results suggest that a stronger immune response in female mice compared with male mice enhances their ability to control parasite replication but increases their morbidity and mortality.
Keywords: IVIS; MCP-1; Toxoplasma gondii; immune endocrine; immune response; parasite burdens; sex differences.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Braun L., Brenier-Pinchart M., Yogavel M., Curt-Varesano A., Curt-Bertini R., Hussain T., Kieffer-Jaquinod S., Coute Y., Pelloux H., Tardieux I., et al. A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J. Exp. Med. 2013;21:2071–2086. doi: 10.1084/jem.20130103. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

