Enteric Tuft Cells in Host-Parasite Interactions
- PMID: 34578195
- PMCID: PMC8467374
- DOI: 10.3390/pathogens10091163
Enteric Tuft Cells in Host-Parasite Interactions
Abstract
Enteric tuft cells are chemosensory epithelial cells gaining attention in the field of host-parasite interactions. Expressing a repertoire of chemosensing receptors and mediators, these cells have the potential to detect lumen-dwelling helminth and protozoan parasites and coordinate epithelial, immune, and neuronal cell defenses against them. This review highlights the versatility of enteric tuft cells and sub-types thereof, showcasing nuances of tuft cell responses to different parasites, with a focus on helminths reflecting the current state of the field. The role of enteric tuft cells in irritable bowel syndrome, inflammatory bowel disease and intestinal viral infection is assessed in the context of concomitant infection with parasites. Finally, the review presents pertinent questions germane to understanding the enteric tuft cell and its role in enteric parasitic infections. There is much to be done to fully elucidate the response of this intriguing cell type to parasitic-infection and there is negligible data on the biology of the human enteric tuft cell-a glaring gap in knowledge that must be filled.
Keywords: Th2 effector; coinfections; epithelial chemosensors; gastrointestinal disorders; helminths; protozoa.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
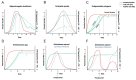
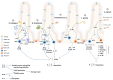
References
-
- Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Döpfer D., Fazil A., Fischer-Walker C.L., Hald T., et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLOS Med. 2015;12:e1001921. doi: 10.1371/journal.pmed.1001921. - DOI - PMC - PubMed
-
- Torgerson P.R., Devleesschauwer B., Praet N., Speybroeck N., Willingham A.L., Kasuga F., Rokni M.B., Zhou X.-N., Fèvre E.M., Sripa B., et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLOS Med. 2015;12:e1001920. doi: 10.1371/journal.pmed.1001920. - DOI - PMC - PubMed
-
- Drake L.J., Jukes M.C.H., Sternberg R.J., Bundy D.A.P. Geohelminth infections (ascariasis, trichuriasis, and hookworm): Cognitive and developmental impacts. Semin. Pediatr. Infect. Dis. 2000;11:245–251. doi: 10.1053/spid.2000.9638. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

