The Molecular Tweezer CLR01 Inhibits Antibody-Resistant Cell-to-Cell Spread of Human Cytomegalovirus
- PMID: 34578265
- PMCID: PMC8472163
- DOI: 10.3390/v13091685
The Molecular Tweezer CLR01 Inhibits Antibody-Resistant Cell-to-Cell Spread of Human Cytomegalovirus
Abstract
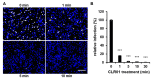
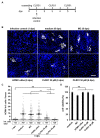
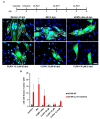
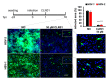
Human cytomegalovirus (HCMV) uses two major ways for virus dissemination: infection by cell-free virus and direct cell-to-cell spread. Neutralizing antibodies can efficiently inhibit infection by cell-free virus but mostly fail to prevent cell-to-cell transmission. Here, we show that the 'molecular tweezer' CLR01, a broad-spectrum antiviral agent, is not only highly active against infection with cell-free virus but most remarkably inhibits antibody-resistant direct cell-to-cell spread of HCMV. The inhibition of cell-to-cell spread by CLR01 was not limited to HCMV but was also shown for the alphaherpesviruses herpes simplex viruses 1 and 2 (HSV-1, -2). CLR01 is a rapid acting small molecule that inhibits HCMV entry at the attachment and penetration steps. Electron microscopy of extracellular virus particles indicated damage of the viral envelope by CLR01, which likely impairs the infectivity of virus particles. The rapid inactivation of viral particles by CLR01, the viral envelope as the main target, and the inhibition of virus entry at different stages are presumably the key to inhibition of cell-free virus infection and cell-to-cell spread by CLR01. Importance: While cell-free spread enables the human cytomegalovirus (HCMV) and other herpesviruses to transmit between hosts, direct cell-to-cell spread is thought to be more relevant for in vivo dissemination within infected tissues. Cell-to-cell spread is resistant to neutralizing antibodies, thus contributing to the maintenance of virus infection and virus dissemination in the presence of an intact immune system. Therefore, it would be therapeutically interesting to target this mode of spread in order to treat severe HCMV infections and to prevent dissemination of virus within the infected host. The molecular tweezer CLR01 exhibits broad-spectrum antiviral activity against a number of enveloped viruses and efficiently blocks antibody-resistant cell-to-cell spread of HCMV, thus representing a novel class of small molecules with promising antiviral activity.
Keywords: CLR01; HCMV; cell-to-cell spread; herpesvirus; inhibition; tweezer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Krawczyk A., Arndt M.A.E., Grosse-Hovest L., Weichert W., Giebel B., Dittmer U., Hengel H., Jäger D., Schneweis K.E., Eis-Hübinger A.M., et al. Overcoming drug-resistant herpes simplex virus (HSV) infection by a humanized antibody. Proc. Natl. Acad. Sci. USA. 2013;110:6760–6765. doi: 10.1073/pnas.1220019110. - DOI - PMC - PubMed
-
- Ganesh L., Leung K., Loré K., Levin R., Panet A., Schwartz O., Koup R.A., Nabel G.J. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol. 2004;78:11980–11987. doi: 10.1128/JVI.78.21.11980-11987.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

