Influenza Antigens NP and M2 Confer Cross Protection to BALB/c Mice against Lethal Challenge with H1N1, Pandemic H1N1 or H5N1 Influenza A Viruses
- PMID: 34578289
- PMCID: PMC8473317
- DOI: 10.3390/v13091708
Influenza Antigens NP and M2 Confer Cross Protection to BALB/c Mice against Lethal Challenge with H1N1, Pandemic H1N1 or H5N1 Influenza A Viruses
Abstract
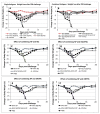
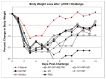
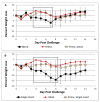
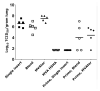
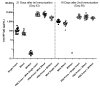
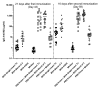
Influenza hemagglutinin (HA) is considered a major protective antigen of seasonal influenza vaccine but antigenic drift of HA necessitates annual immunizations using new circulating HA versions. Low variation found within conserved non-HA influenza virus (INFV) antigens may maintain protection with less frequent immunizations. Conserved antigens of influenza A virus (INFV A) that can generate cross protection against multiple INFV strains were evaluated in BALB/c mice using modified Vaccinia virus Ankara (MVA)-vectored vaccines that expressed INFV A antigens hemagglutinin (HA), matrix protein 1 (M1), nucleoprotein (NP), matrix protein 2 (M2), repeats of the external portion of M2 (M2e) or as tandem repeats (METR), and M2e with transmembrane region and cytoplasmic loop (M2eTML). Protection by combinations of non-HA antigens was equivalent to that of subtype-matched HA. Combinations of NP and forms of M2e generated serum antibody responses and protected mice against lethal INFV A challenge using PR8, pandemic H1N1 A/Mexico/4108/2009 (pH1N1) or H5N1 A/Vietnam/1203/2004 (H5N1) viruses, as demonstrated by reduced lung viral burden and protection against weight loss. The highest levels of protection were obtained with NP and M2e antigens delivered as MVA inserts, resulting in broadly protective immunity in mice and enhancement of previous natural immunity to INFV A.
Keywords: conserved antigens; hemagglutinin; influenza A; matrix protein 1; matrix protein 2; modified Vaccinia virus Ankara (MVA); nucleoprotein; vaccine.
Conflict of interest statement
All authors are present or former employees of Emergent BioSolutions. J.R.I., T.E.H. and H.L. own Emergent stock. All other authors have no competing interest.
Figures

References
-
- Xie H., Li X., Gao J., Lin Z., Jing X., Plant E., Zoueva O., Eichelberger M.C., Ye Z. Revisiting the 1976 "swine flu" vaccine clinical trials: Cross-reactive hemagglutinin and neuraminidase antibodies and their role in protection against the 2009 H1N1 pandemic virus in mice. Clin. Infect. Dis. 2011;53:1179–1187. doi: 10.1093/cid/cir693. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

