Clinical Evaluation of In-House-Produced 3D-Printed Nasopharyngeal Swabs for COVID-19 Testing
- PMID: 34578334
- PMCID: PMC8473445
- DOI: 10.3390/v13091752
Clinical Evaluation of In-House-Produced 3D-Printed Nasopharyngeal Swabs for COVID-19 Testing
Abstract
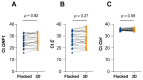
3D-printed alternatives to standard flocked swabs were rapidly developed to provide a response to the unprecedented and sudden need for an exponentially growing amount of diagnostic tools to fight the COVID-19 pandemic. In light of the anticipated shortage, a hospital-based 3D-printing platform was implemented in our institution for the production of swabs for nasopharyngeal and oropharyngeal sampling based on the freely available, open-source design provided to the community by University of South Florida's Health Radiology and Northwell Health System teams as a replacement for locally used commercial swabs. Validation of our 3D-printed swabs was performed with a head-to-head diagnostic accuracy study of the 3D-printed "Northwell model" with the cobas PCR Media® swab sample kit. We observed an excellent concordance (total agreement 96.8%, Kappa 0.936) in results obtained with the 3D-printed and flocked swabs, indicating that the in-house 3D-printed swab could be used reliably in the context of a shortage of flocked swabs. To our knowledge, this is the first study to report on autonomous hospital-based production and clinical validation of 3D-printed swabs.
Keywords: 3D-printed nasopharyngeal swabs; COVID-19; PCR; SARS-CoV-2; diagnosis.
Conflict of interest statement
S.G.L. has received funding from Roche Diagnosis unrelated to the present study to test PCR medium stability. F.C. received grants paid to the organization for research projects unrelated to the present study from Roche Diagnostics and Merck Sharp and Dome, honorariums for presentations from Merck Sharp and Dome and Roche Diagnostics, and has participated in an expert vaccine group formed by Merck Sharp and Dome.
Figures


Similar articles
-
Prospective clinical validation of 3D printed nasopharyngeal swabs for diagnosis of COVID-19.Diagn Microbiol Infect Dis. 2021 Mar;99(3):115257. doi: 10.1016/j.diagmicrobio.2020.115257. Epub 2020 Oct 22. Diagn Microbiol Infect Dis. 2021. PMID: 33220640 Free PMC article.
-
3-Dimensional Printed Alternative to the Standard Synthetic Flocked Nasopharyngeal Swabs Used for Coronavirus Disease 2019 Testing.Clin Infect Dis. 2021 Nov 2;73(9):e3027-e3032. doi: 10.1093/cid/ciaa1366. Clin Infect Dis. 2021. PMID: 32910817 Free PMC article. Clinical Trial.
-
Pandemic printing: a novel 3D-printed swab for detecting SARS-CoV-2.Med J Aust. 2020 Sep;213(6):276-279. doi: 10.5694/mja2.50726. Epub 2020 Aug 9. Med J Aust. 2020. PMID: 32772375 Free PMC article.
-
Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis.J Med Virol. 2021 Feb;93(2):719-725. doi: 10.1002/jmv.26349. Epub 2020 Aug 2. J Med Virol. 2021. PMID: 32706393 Free PMC article.
-
Relative sensitivity of anterior nares and nasopharyngeal swabs for initial detection of SARS-CoV-2 in ambulatory patients: Rapid review and meta-analysis.PLoS One. 2021 Jul 20;16(7):e0254559. doi: 10.1371/journal.pone.0254559. eCollection 2021. PLoS One. 2021. PMID: 34283845 Free PMC article. Review.
Cited by
-
Original article: novelty of Canadian manufacture nasopharyngeal swabs for collection of samples being tested for SARS-CoV-2 in a pandemic setting.Front Public Health. 2024 May 9;12:1344295. doi: 10.3389/fpubh.2024.1344295. eCollection 2024. Front Public Health. 2024. PMID: 38784579 Free PMC article.
-
CFD based analysis of 3D printed nasopharyngeal swabs for COVID-19 diagnostics.Comput Methods Programs Biomed. 2022 Aug;223:106977. doi: 10.1016/j.cmpb.2022.106977. Epub 2022 Jun 27. Comput Methods Programs Biomed. 2022. PMID: 35780521 Free PMC article.
-
Clinical validation of 3D-printed swabs in adults and children for SARS-CoV-2 detection.Biol Methods Protoc. 2023 Jun 7;8(1):bpad009. doi: 10.1093/biomethods/bpad009. eCollection 2023. Biol Methods Protoc. 2023. PMID: 37351376 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
References
-
- Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. The Novel Coronavirus, 2019-nCoV, is Highly Contagious and More Infectious Than Initially Estimated. medRxiv. 2020 doi: 10.1101/2020.02.07.20021154. - DOI
-
- World Health Organization WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. [(accessed on 1 March 2021)]. Available online: https://www.who.int/director-general/speeches/detail/who-director-genera....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

