Treatments for HBV: A Glimpse into the Future
- PMID: 34578347
- PMCID: PMC8473442
- DOI: 10.3390/v13091767
Treatments for HBV: A Glimpse into the Future
Abstract
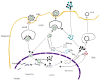
The hepatitis B virus is responsible for most of the chronic liver disease and liver cancer worldwide. As actual therapeutic strategies have had little success in eradicating the virus from hepatocytes, and as lifelong treatment is often required, new drugs targeting the various phases of the hepatitis B virus (HBV) lifecycle are currently under investigation. In this review, we provide an overview of potential future treatments for HBV.
Keywords: HBV; direct antiviral agents; gene therapy; immunotherapy; therapeutic vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- WHO|Global Hepatitis Report. 2017. [(accessed on 30 March 2021)]. Available online: http://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/
-
- Ni Y., Lempp F.A., Mehrle S., Nkongolo S., Kaufman C., Fälth M., Stindt J., Königer C., Nassal M., Kubitz R., et al. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology. 2014;146:1070–1083. doi: 10.1053/j.gastro.2013.12.024. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

