Green Synthesis of Cu Nanoparticles in Modulating the Reactivity of Amine-Functionalized Composite Materials towards Cross-Coupling Reactions
- PMID: 34578576
- PMCID: PMC8464933
- DOI: 10.3390/nano11092260
Green Synthesis of Cu Nanoparticles in Modulating the Reactivity of Amine-Functionalized Composite Materials towards Cross-Coupling Reactions
Abstract
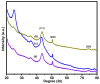
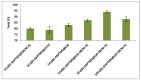
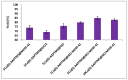
Control over both dispersion and the particle size distribution of supported metal particles is of paramount importance for the catalytic activity of composite materials. We describe the synthesis of materials with Cu nanoparticles well-dispersed on different amine-functionalized supports, using the extract of Wallich Spurge as a green, reducing agent. Graphene oxide (GO), mesoporous silica (MCM-41), mesoporous zirconia, and reduced graphene oxide-mesoporous silica (RGO-MCM-41) were explored as supports. Cu nanoparticles were better stabilized on RGO-MCM-41 compared to other supports. The novel composite materials were characterized by X-ray diffraction (XRD), Raman spectra, Scanning electron microscope (SEM), Transmission electron microscopy analysis and HR-TEM. SEM and EDX techniques. High angle XRD confirmed the conversion of graphene oxide to reduced graphene oxide (RGO) with plant extract as a reducing agent. Both XRD and TEM techniques confirmed the Cu nanoparticle formation. The catalytic activity of all the prepared materials for the Ullmann coupling reactions of carbon-, oxygen-, and nitrogen-containing nucleophiles with iodobenzene was evaluated. From the results, 5 wt% Cu on amine-functionalized reduced graphene oxide/mesoporous silica nanocomposite (5 wt%Cu(0)-AAPTMS@RGO-MCM-41) exhibited excellent efficiency with 97% yield of the C-C coupling product in water at 80 °C in 5 h. The activity remained unaltered almost up to the fourth cycle. The Cu nanoparticles stabilized by organic amine group on RGO hybrid facilitated sustained activity.
Keywords: C-C coupling; C-N coupling; C-O coupling; amine functionalized; reduced graphene oxide composite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Gawande M.B., Branco P., Parghi K.D., Shrikhande J.J., Pandey R.K., Ghumman C.A.A., Bundaleski N., Teodoro O., Jayaram R.V. Synthesis and characterization of versatile MgO–ZrO2 mixed metal oxide nanoparticles and their applications. Catal. Sci. Technol. 2011;1:1653–1664. doi: 10.1039/c1cy00259g. - DOI
LinkOut - more resources
Full Text Sources

