Study on the Efficacy and Mechanism of Lycium barbarum Polysaccharide against Lead-Induced Renal Injury in Mice
- PMID: 34578823
- PMCID: PMC8470764
- DOI: 10.3390/nu13092945
Study on the Efficacy and Mechanism of Lycium barbarum Polysaccharide against Lead-Induced Renal Injury in Mice
Abstract
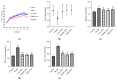
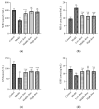
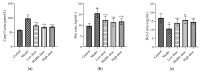
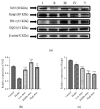
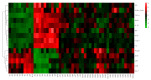
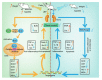
Lead is one of the most common heavy metal pollutants in the environment. Prolonged exposure to lead will induce oxidative stress, inflammation, and apoptosis in the kidneys, which in turn causes kidney injury. Lycium barbarum polysaccharide (LBP) is well known for its numerous pharmacological properties. This study aims to explore the efficacy and mechanism of LBP against lead-induced kidney damage in mice. Symptoms of renal injury were induced in mice by using 25 mg/kg lead acetate (PbAc2), and different doses of LBP (200, 400, and 600 mg/kg BW) were orally administrated to PbAc2-treated mice for five weeks. The results of the pharmacodynamics experiment showed that the renal pathological damages, serum creatinine (Cre), blood urea nitrogen (BUN), and kidney index of PbAc2-treated mice could be significantly alleviated by treatment with LBP. Further, LBP treatment significantly increased the weight and feed intake of PbAc2-treated mice. The dose effect results indicated that a medium dose of LBP was superior to high and low doses. The results of mechanistic experiments showed that LBP could attenuate oxidative stress, inflammation, and apoptosis in the kidneys of mice with lead toxicity by activating the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway.
Keywords: Lycium barbarum polysaccharide; Nrf2 signaling pathway; apoptosis; inflammatory; lead-induced renal injury; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Lycium barbarum polysaccharides attenuate cardiovascular oxidative stress injury by enhancing the Keap1/Nrf2 signaling pathway in exhaustive exercise rats.Mol Med Rep. 2021 Sep;24(3):643. doi: 10.3892/mmr.2021.12282. Epub 2021 Jul 19. Mol Med Rep. 2021. PMID: 34278476
-
Intervention effect of Lycium barbarum polysaccharide on lead-induced kidney injury mice and its mechanism: A study based on the PI3K/Akt/mTOR signaling pathway.J Ethnopharmacol. 2024 Jan 30;319(Pt 2):117197. doi: 10.1016/j.jep.2023.117197. Epub 2023 Sep 16. J Ethnopharmacol. 2024. PMID: 37722516
-
Activation of the Nrf2/HO-1 antioxidant pathway contributes to the protective effects of Lycium barbarum polysaccharides in the rodent retina after ischemia-reperfusion-induced damage.PLoS One. 2014 Jan 6;9(1):e84800. doi: 10.1371/journal.pone.0084800. eCollection 2014. PLoS One. 2014. PMID: 24400114 Free PMC article.
-
Neuro-protective Mechanisms of Lycium barbarum.Neuromolecular Med. 2016 Sep;18(3):253-63. doi: 10.1007/s12017-016-8393-y. Epub 2016 Mar 31. Neuromolecular Med. 2016. PMID: 27033360 Review.
-
Effect of Lycium barbarum polysaccharides on cell signal transduction pathways.Biomed Pharmacother. 2022 Mar;147:112620. doi: 10.1016/j.biopha.2022.112620. Epub 2022 Jan 12. Biomed Pharmacother. 2022. PMID: 35032768 Review.
Cited by
-
Renal Health Through Medicine-Food Homology: A Comprehensive Review of Botanical Micronutrients and Their Mechanisms.Nutrients. 2024 Oct 18;16(20):3530. doi: 10.3390/nu16203530. Nutrients. 2024. PMID: 39458524 Free PMC article. Review.
-
The effect of dietary supplementation of Lycium barbarum leaves on the growth performance, organ indexes and intestinal microflora of rats.Front Vet Sci. 2024 Jul 31;11:1416793. doi: 10.3389/fvets.2024.1416793. eCollection 2024. Front Vet Sci. 2024. PMID: 39144075 Free PMC article.
-
Functional and structural dissection of glycosyltransferases underlying the glycodiversity of wolfberry-derived bioactive ingredients lycibarbarspermidines.Nat Commun. 2024 May 30;15(1):4588. doi: 10.1038/s41467-024-49010-9. Nat Commun. 2024. PMID: 38816433 Free PMC article.
-
Lycium barbarum polysaccharide alleviates dextran sodium sulfate-induced inflammatory bowel disease by regulating M1/M2 macrophage polarization via the STAT1 and STAT6 pathways.Front Pharmacol. 2023 Apr 18;14:1044576. doi: 10.3389/fphar.2023.1044576. eCollection 2023. Front Pharmacol. 2023. PMID: 37144216 Free PMC article.
-
Heavy Metal Exposure: Molecular Pathways, Clinical Implications, and Protective Strategies.Antioxidants (Basel). 2024 Jan 5;13(1):76. doi: 10.3390/antiox13010076. Antioxidants (Basel). 2024. PMID: 38247500 Free PMC article. Review.
References
-
- Gloaguen T.V., Motta P.N.S.D., Couto C.F. A grain-size correction for metal pollution indexes in river sediments. Int. J. Sediment Res. 2020;36:362–372. doi: 10.1016/j.ijsrc.2020.10.005. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

