The Protective Effect of Oral Application of Corni Fructus on the Disorders of the Cornea, Conjunctiva, Lacrimal Gland and Retina by Topical Particulate Matter 2.5
- PMID: 34578864
- PMCID: PMC8464674
- DOI: 10.3390/nu13092986
The Protective Effect of Oral Application of Corni Fructus on the Disorders of the Cornea, Conjunctiva, Lacrimal Gland and Retina by Topical Particulate Matter 2.5
Abstract
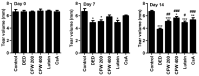
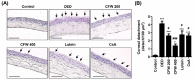
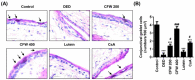
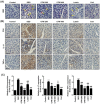
Particulate matter 2.5 (PM2.5) may aggravate dry eye disease (DED). Corni Fructus (CF), which is fruit of Cornus officinalis Sieb. et Zucc., has been reported to have various beneficial pharmacological effects, whereas the effect of CF on the eye is still unknown. Therefore, in this study, we investigated the effect of oral administration of water extract of CF (CFW) on the eye, hematology, and biochemistry in a DED model induced by topical exposure to PM2.5. Furthermore, the efficacy of CFW compared with cyclosporine (CsA), an anti-inflammatory agent, and lutein, the posterior eye-protective agent. Sprague-Dawley rats were topically administered 5 mg/mL PM2.5 in both eyes four times daily for 14 days. During the same period, CFW (200 mg/kg and 400 mg/kg) and lutein (4.1 mg/kg) were orally administered once a day. All eyes of rats in the 0.05% cyclosporine A (CsA)-treated group were topically exposed to 20 μL of CsA, twice daily for 14 days. Oral administration of CFW attenuated the PM2.5-induced reduction of tear secretion and corneal epithelial damage. In addition, CFW protected against goblet cell loss in conjunctiva and overexpression of inflammatory factors in the lacrimal gland following topical exposure to PM2.5. Furthermore, CFW markedly prevented PM2.5-induced ganglion cell loss and recovered the thickness of inner plexiform layer. Meanwhile, CFW treatment decreased the levels of total cholesterol and low-density lipoprotein cholesterol in serum induced by PM2.5. Importantly, the efficacy of CFW was superior or similar to that of CsA and lutein. Taken together, oral administration of CFW may have protective effects against PM2.5-induced DED symptoms via stabilization of the tear film and suppression of inflammation. Furthermore, CFW may in part contribute to improving retinal function and lipid metabolism disorder.
Keywords: Corni Fructus; cyclosporine A; dry eye disease; goblet cells; lutein; particulate matter 2.5; retinal ganglion cells; tear production.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
Similar articles
-
Achyranthis radix Extract Improves Urban Particulate Matter-Induced Dry Eye Disease.Int J Environ Res Public Health. 2019 Sep 4;16(18):3229. doi: 10.3390/ijerph16183229. Int J Environ Res Public Health. 2019. PMID: 31487776 Free PMC article.
-
The Protective Effect of Topical Spermidine on Dry Eye Disease with Retinal Damage Induced by Diesel Particulate Matter2.5.Pharmaceutics. 2021 Sep 10;13(9):1439. doi: 10.3390/pharmaceutics13091439. Pharmaceutics. 2021. PMID: 34575516 Free PMC article.
-
Effect of Aucubin-Containing Eye Drops on Tear Hyposecretion and Lacrimal Gland Damage Induced by Urban Particulate Matter in Rats.Molecules. 2022 May 4;27(9):2926. doi: 10.3390/molecules27092926. Molecules. 2022. PMID: 35566278 Free PMC article.
-
Is the main lacrimal gland indispensable? Contributions of the corneal and conjunctival epithelia.Surv Ophthalmol. 2016 Sep-Oct;61(5):616-27. doi: 10.1016/j.survophthal.2016.02.006. Epub 2016 Mar 9. Surv Ophthalmol. 2016. PMID: 26968256 Review.
-
Corni Fructus: a review of chemical constituents and pharmacological activities.Chin Med. 2018 Jun 25;13:34. doi: 10.1186/s13020-018-0191-z. eCollection 2018. Chin Med. 2018. PMID: 29983732 Free PMC article. Review.
Cited by
-
Effects of Different Fermentation and Clarification Methods on the Color, Physicochemical Characteristics, and Aroma Profile of Healthcare Cornus-Kiwifruit Composite Wine.Foods. 2025 May 11;14(10):1705. doi: 10.3390/foods14101705. Foods. 2025. PMID: 40428485 Free PMC article.
-
Cornuside Is a Potential Agent against Alzheimer's Disease via Orchestration of Reactive Astrocytes.Nutrients. 2022 Aug 3;14(15):3179. doi: 10.3390/nu14153179. Nutrients. 2022. PMID: 35956355 Free PMC article.
-
Roles and Mechanisms of Regulated Necrosis in Corneal Diseases: Progress and Perspectives.J Ophthalmol. 2022 May 23;2022:2695212. doi: 10.1155/2022/2695212. eCollection 2022. J Ophthalmol. 2022. PMID: 35655803 Free PMC article. Review.
-
Pathological Mechanisms of Particulate Matter-Mediated Ocular Disorders: A Review.Int J Mol Sci. 2024 Nov 11;25(22):12107. doi: 10.3390/ijms252212107. Int J Mol Sci. 2024. PMID: 39596177 Free PMC article. Review.
-
Using Network Pharmacology and Molecular Docking to Explore the Mechanism of Qiju Dihuang Pill against Dry Eye Disease.Comput Math Methods Med. 2022 Dec 22;2022:7316794. doi: 10.1155/2022/7316794. eCollection 2022. Comput Math Methods Med. 2022. PMID: 36590763 Free PMC article.
References
-
- Kang W.S., Choi H., Jang G., Lee K.H., Kim E., Kim K.J., Jeong G.Y., Kim J.S., Na C.S., Kim S. Long-term exposure to urban particulate matter on the ocular surface and the incidence of deleterious changes in the cornea, conjunctiva and retina in rats. Int. J. Mol. Sci. 2020;21:4976. doi: 10.3390/ijms21144976. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

