Ferulic Acid Metabolites Attenuate LPS-Induced Inflammatory Response in Enterocyte-like Cells
- PMID: 34579029
- PMCID: PMC8471535
- DOI: 10.3390/nu13093152
Ferulic Acid Metabolites Attenuate LPS-Induced Inflammatory Response in Enterocyte-like Cells
Abstract
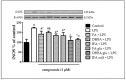
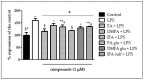
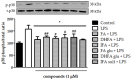
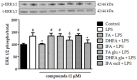
Ferulic acid (FA) is a polyphenol pertaining to the class of hydroxycinnamic acids present in numerous foods of a plant origin. Its dietary consumption leads to the formation of several phase I and II metabolites in vivo, which represent the largest amount of ferulates in the circulation and in the intestine in comparison with FA itself. In this work, we evaluated their efficacy against the proinflammatory effects induced by lipopolysaccharide (LPS) in intestinal Caco-2 cell monolayers, as well as the mechanisms underlying their protective action. LPS-induced overexpression of proinflammatory enzymes such as inducible nitric oxide synthase (iNOS) and the consequent hyperproduction of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) were limited by physiological relevant concentrations (1 µM) of FA, its derivatives isoferulic acid (IFA) and dihydroferulic acid (DHFA), and their glucuronidated and sulfated metabolites, which acted upstream by limiting the activation of MAPK p38 and ERK and of Akt kinase, thus decreasing the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB) translocation into the nucleus. Furthermore, the compounds were found to promote the expression of Nrf2, which may have contributed to the downregulation of NF-ĸB activity. The overall data show that phase I/II metabolites retain the efficacy of their dietary free form in contrasting inflammatory response.
Keywords: MAP kinases; inflammation; intestinal cells; lipopolysaccharide; metabolites; nitric oxide; nitric oxide synthase; polyphenols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

