Rhabdomyolysis Following Ad26.COV2.S COVID-19 Vaccination
- PMID: 34579193
- PMCID: PMC8472996
- DOI: 10.3390/vaccines9090956
Rhabdomyolysis Following Ad26.COV2.S COVID-19 Vaccination
Abstract
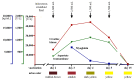
We report the case of a 19-year-old male who complained of myalgia, muscle weakness, and darkened urine two days after receiving his Ad26.COV2.S (Johnson & Johnson, New Brunswick, New Jersey, United States) COVID-19 vaccination. Blood examination revealed an increased creatine kinase (CK) level, and his urinary dipstick tested positive for blood, indicative of acute rhabdomyolysis. Serum creatinine levels were normal. Rhabdomyolysis due to strenuous physical activity was ruled out and further diagnostics excluded an autoimmune cause. Under repeated treatment with intravenous fluid resuscitation (outpatient treatment), his symptoms resolved and peak CK levels of 44,180 U/L returned to almost normal levels within two weeks. Rhabdomyolysis is a rare, potentially fatal vaccine-induced reaction. Further research is needed to better understand the underlying pathomechanism and to investigate whether subcutaneous injection of vaccines may be able to prevent rhabdomyolysis.
Keywords: Ad26.COV2.S; COVID-19; myoglobinuria; rhabdomyolysis; vaccine.
Conflict of interest statement
G.G. was supported by a grant from the Austrian Federal Ministry of Education, Science and Research (ACOVACT). The funding had no role in data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare that there are no competing interests.
Figures
References
-
- Bos R., Rutten L., van der Lubbe J.E., Bakkers M.J., Hardenberg G., Wegmann F., Zuijdgeest D., De Wilde A.H., Koornneef A., Verwillingen A., et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5:91. doi: 10.1038/s41541-020-00243-x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials