Safety and Efficacy of Spray Intranasal Live Attenuated Influenza Vaccine: Systematic Review and Meta-Analysis
- PMID: 34579235
- PMCID: PMC8472940
- DOI: 10.3390/vaccines9090998
Safety and Efficacy of Spray Intranasal Live Attenuated Influenza Vaccine: Systematic Review and Meta-Analysis
Abstract
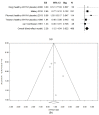
Although influenza is a major public health concern, little is known about the use of spray live attenuated influenza vaccine (LAIV) among adults. For this reason, we conducted a systematic review and meta-analysis to investigate the efficacy and safety of LAIV, especially in adults with/without clinical conditions and children <2 years, with the final aim of possibly extending the clinical indications. PubMed/MEDLINE and Scopus were the two databases consulted through February 2021. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed. A critical appraisal was conducted. Analyses were performed by using ProMeta3 software. Twenty-two studies were included, showing that LAIV was associated with a higher probability of seroconversion when compared with a placebo and considering the A/H1N1 serotype (pooled OR = 2.26 (95% CI = 1.12-4.54), p-value = 0.022; based on 488 participants, without heterogeneity (I2 = 0.0%)). The meta-analysis also confirmed no significant association with systemic adverse events. Only rhinorrhea, nasal congestion, and sore throat were significantly associated with LAIV compared to the placebo. Despite limited available evidence, LAIV has proved to be a safe and effective flu vaccination, also due to its very low invasiveness, and our review's results can be considered a starting point for guiding future research and shaping forthcoming vaccination campaigns.
Keywords: adult; antibody response; immune response; immunogenicity; inactivated influenza vaccine; infant; intranasal live attenuated influenza vaccine; safety.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Troeger C.E., Blacker B.F., Khalil I.A., Zimsen S.R., Albertson S.B., Abate D., Abdela J., Adhikari T.B., Aghayan S.A., Agrawal S., et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: An analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2019;7:69–89. doi: 10.1016/S2213-2600(18)30496-X. - DOI - PMC - PubMed
-
- Grohskopf L.A., Alyanak E., Broder K.R., Blanton L.H., Fry A.M., Jernigan D.B., Atmar R.L. Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices—United States, 2020–2021 influenza season. MMWR Recomm. Rep. 2020;69:1–24. doi: 10.15585/mmwr.rr6908a1. - DOI - PMC - PubMed
-
- Odone A., Chiesa V., Ciorba V., Cella P., Pasquarella C., Signorelli C. Influenza and immunization: A quantitative study of media coverage in the season of the “Fluad case”. Epidemiol. Prev. 2015;39:139–145. - PubMed

