Varicella Zoster Virus Reactivation Following COVID-19 Vaccination: A Systematic Review of Case Reports
- PMID: 34579250
- PMCID: PMC8471236
- DOI: 10.3390/vaccines9091013
Varicella Zoster Virus Reactivation Following COVID-19 Vaccination: A Systematic Review of Case Reports
Abstract
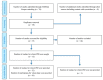
The newly developed COVID-19 vaccines have established a safe profile, yet some individuals experience a wide range of adverse events. Recently, reactivation of varicella zoster virus (VZV) has been observed after administration of different COVID-19 vaccines, although causality remains a matter of debate. The aim of this systematic review was to examine the available literature and provide an overview of reported cases of VZV reactivation following COVID-19 vaccination. We identified 12 eligible articles which included 91 patients with herpes zoster (HZ) following COVID-19 vaccination. Hypertension was the main comorbidity present in 18% of patients (16/91). Additionally, 13% of patients (12/91) had an autoimmune condition with rheumatoid arthritis being the most common (4/12). Moreover, 10% of patients (9/91) were receiving immunosuppressants. The dermatomal distribution of skin lesions varied among patients, with the mammary region being most affected. On average, symptoms developed 5.8 days post-vaccination irrespective of dose and treatment with oral valacyclovir as a monotherapy was employed in most patients (23/91). HZ is possibly a condition clinicians may expect to encounter in patients receiving COVID-19 vaccines. While causality has not yet been established increased awareness and early recognition of the disorder would be crucial for the optimal management of these patients.
Keywords: COVID-19 vaccine; SARS-CoV-2; Varicella zoster virus; case reports; case series; coronavirus; herpes zoster; systematic review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. - DOI - PMC - PubMed
-
- Johnson J. Johnson & Johnson COVID-19 Vaccine Authorized by US FDA For Emergency Use-First Single-Shot Vaccine in Fight Against Global Pandemic. Johnson & Johnson; New Brunswick, NJ, USA: 2021.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous