The Burkholderia cenocepacia Type VI Secretion System Effector TecA Is a Virulence Factor in Mouse Models of Lung Infection
- PMID: 34579569
- PMCID: PMC8546862
- DOI: 10.1128/mBio.02098-21
The Burkholderia cenocepacia Type VI Secretion System Effector TecA Is a Virulence Factor in Mouse Models of Lung Infection
Abstract
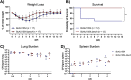
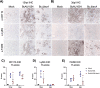
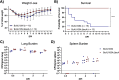
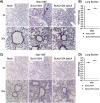
Burkholderia cenocepacia is a member of the Burkholderia cepacia complex (Bcc), a group of bacteria with members responsible for causing lung infections in cystic fibrosis (CF) patients. The most severe outcome of Bcc infection in CF patients is cepacia syndrome, a disease characterized by necrotizing pneumonia with bacteremia and sepsis. B. cenocepacia is strongly associated with cepacia syndrome, making it one of the most virulent members of the Bcc. Mechanisms underlying the pathogenesis of B. cenocepacia in lung infections and cepacia syndrome remain to be uncovered. B. cenocepacia is primarily an intracellular pathogen and encodes the type VI secretion system (T6SS) effector TecA, which is translocated into host phagocytes. TecA is a deamidase that inactivates multiple Rho GTPases, including RhoA. Inactivation of RhoA by TecA triggers assembly of the pyrin inflammasome, leading to secretion of proinflammatory cytokines, such as interleukin-1β, from macrophages. Previous work with the B. cenocepacia clinical isolate J2315 showed that TecA increases immunopathology during acute lung infection in C57BL/6 mice and suggested that this effector acts as a virulence factor by triggering assembly of the pyrin inflammasome. Here, we extend these results using a second B. cenocepacia clinical isolate, AU1054, to demonstrate that TecA exacerbates weight loss and lethality during lung infection in C57BL/6 mice and mice engineered to have a CF genotype. Unexpectedly, pyrin was dispensable for TecA virulence activity in both mouse infection models. Our findings establish that TecA is a B. cenocepacia virulence factor that exacerbates lung inflammation, weight loss, and lethality in mouse infection models. IMPORTANCE B. cenocepacia is often considered the most virulent species in the Bcc because of its close association with cepacia syndrome in addition to its capacity to cause chronic lung infections in CF patients (1). Prior to the current study, virulence factors of B. cenocepacia important for causing lethal disease had not been identified in a CF animal model of lung infection. Results of this study describe a CF mouse model and its use in demonstrating that the T6SS effector TecA of B. cenocepacia exacerbates inflammatory cell recruitment and weight loss and is required for lethality and, thus, acts as a key virulence factor during lung infection. This model will be important in further studies to better understand TecA's role as a virulence factor and in investigating ways to prevent or treat B. cenocepacia infections in CF patients. Additionally, TecA may be the founding member of a family of virulence factors in opportunistic pathogens.
Keywords: Burkholderia cenocepacia; lung infection; type VI secretion.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
