Effectiveness of anisodamine for the treatment of critically ill patients with septic shock: a multicentre randomized controlled trial
- PMID: 34579741
- PMCID: PMC8474812
- DOI: 10.1186/s13054-021-03774-4
Effectiveness of anisodamine for the treatment of critically ill patients with septic shock: a multicentre randomized controlled trial
Abstract
Background: Septic shock is characterized by an uncontrolled inflammatory response and microcirculatory dysfunction. There is currently no specific agent for treating septic shock. Anisodamine is an agent extracted from traditional Chinese medicine with potent anti-inflammatory effects. However, its clinical effectiveness remains largely unknown.
Methods: In a multicentre, open-label trial, we randomly assigned adults with septic shock to receive either usual care or anisodamine (0.1-0.5 mg per kilogram of body weight per hour), with the anisodamine doses adjusted by clinicians in accordance with the patients' shock status. The primary end point was death on hospital discharge. The secondary end points were ventilator-free days at 28 days, vasopressor-free days at 28 days, serum lactate and sequential organ failure assessment (SOFA) score from days 0 to 6. The differences in the primary and secondary outcomes were compared between the treatment and usual care groups with the χ2 test, Student's t test or rank-sum test, as appropriate. The false discovery rate was controlled for multiple testing.
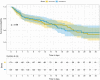
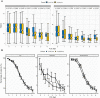
Results: Of the 469 patients screened, 355 were assigned to receive the trial drug and were included in the analyses-181 patients received anisodamine, and 174 were in the usual care group. We found no difference between the usual care and anisodamine groups in hospital mortality (36% vs. 30%; p = 0.348), or ventilator-free days (median [Q1, Q3], 24.4 [5.9, 28] vs. 26.0 [8.5, 28]; p = 0.411). The serum lactate levels were significantly lower in the treated group than in the usual care group after day 3. Patients in the treated group were less likely to receive vasopressors than those in the usual care group (OR [95% CI] 0.84 [0.50, 0.93] for day 5 and 0.66 [0.37, 0.95] for day 6).
Conclusions: There is no evidence that anisodamine can reduce hospital mortality among critically ill adults with septic shock treated in the intensive care unit. Trial registration ClinicalTrials.gov ( NCT02442440 ; Registered on 13 April 2015).
Keywords: Anisodamine; Mechanical ventilation; Mortality; Randomized controlled trial; Septic shock.
© 2021. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures

Comment in
-
Anisodamine microcirulatory effects in septic shock: be aware of cardiac side effects.Crit Care. 2021 Dec 16;25(1):433. doi: 10.1186/s13054-021-03854-5. Crit Care. 2021. PMID: 34915896 Free PMC article. No abstract available.
References
-
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Rhodes Kievlan D, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Articles 200 www.thelancet.com. 2020;395. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

