The Nereid on the rise: Platynereis as a model system
- PMID: 34579780
- PMCID: PMC8477482
- DOI: 10.1186/s13227-021-00180-3
The Nereid on the rise: Platynereis as a model system
Abstract
The Nereid Platynereis dumerilii (Audouin and Milne Edwards (Annales des Sciences Naturelles 1:195-269, 1833) is a marine annelid that belongs to the Nereididae, a family of errant polychaete worms. The Nereid shows a pelago-benthic life cycle: as a general characteristic for the superphylum of Lophotrochozoa/Spiralia, it has spirally cleaving embryos developing into swimming trochophore larvae. The larvae then metamorphose into benthic worms living in self-spun tubes on macroalgae. Platynereis is used as a model for genetics, regeneration, reproduction biology, development, evolution, chronobiology, neurobiology, ecology, ecotoxicology, and most recently also for connectomics and single-cell genomics. Research on the Nereid started with studies on eye development and spiralian embryogenesis in the nineteenth and early twentieth centuries. Transitioning into the molecular era, Platynereis research focused on posterior growth and regeneration, neuroendocrinology, circadian and lunar cycles, fertilization, and oocyte maturation. Other work covered segmentation, photoreceptors and other sensory cells, nephridia, and population dynamics. Most recently, the unique advantages of the Nereid young worm for whole-body volume electron microscopy and single-cell sequencing became apparent, enabling the tracing of all neurons in its rope-ladder-like central nervous system, and the construction of multimodal cellular atlases. Here, we provide an overview of current topics and methodologies for P. dumerilii, with the aim of stimulating further interest into our unique model and expanding the active and vibrant Platynereis community.
Keywords: Annelida; Evo-devo; Integrative biology; Marine model species; Spiralia.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Ocean Biodiversity Information System. https://obis.org/. Accessed 2 Apr 2021.
-
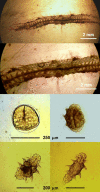
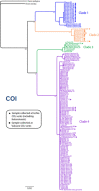
- Teixeira MAL, Nygren A, Ravara A, Vieira PE, Hernández JC, Costa FO. The small polychaete Platynereis dumerilii revealed as a large species complex with fourteen MOTUs in European marine habitats. Eur Surg. 2021;3(4):e64937.
-
- Wäge J, Valvassori G, Hardege JD, Schulze A, Gambi MC. The sibling polychaetes Platynereis dumerilii and Platynereis massiliensis in the Mediterranean Sea: are phylogeographic patterns related to exposure to ocean acidification? Mar Biol. 2017;164(10):199. doi: 10.1007/s00227-017-3222-x. - DOI
-
- Kisseleva MI. Dynamique et production de la population de Polychète Platynereis dumerilii dans la biocoenose de la Cystoseira en Mer Noire. “Grigore Antipa”. Trav Mus Natl Hist Nat Grigore Antipa. 1971;11:49–58.
-
- Popa LO, Popa OP, Krapal A-M, Iorgu EI, Surugiu V. Fine-scale population genetics analysis of Platynereis dumerilii (Polychaeta, Nereididae) in the Black Sea: how do local marine currents drive geographical differentiation? J Exp Zool A Ecol Genet Physiol. 2014;321(1):41–47. doi: 10.1002/jez.1835. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

