Deep learning in cancer diagnosis, prognosis and treatment selection
- PMID: 34579788
- PMCID: PMC8477474
- DOI: 10.1186/s13073-021-00968-x
Deep learning in cancer diagnosis, prognosis and treatment selection
Abstract
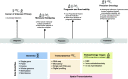
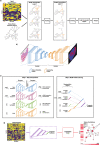
Deep learning is a subdiscipline of artificial intelligence that uses a machine learning technique called artificial neural networks to extract patterns and make predictions from large data sets. The increasing adoption of deep learning across healthcare domains together with the availability of highly characterised cancer datasets has accelerated research into the utility of deep learning in the analysis of the complex biology of cancer. While early results are promising, this is a rapidly evolving field with new knowledge emerging in both cancer biology and deep learning. In this review, we provide an overview of emerging deep learning techniques and how they are being applied to oncology. We focus on the deep learning applications for omics data types, including genomic, methylation and transcriptomic data, as well as histopathology-based genomic inference, and provide perspectives on how the different data types can be integrated to develop decision support tools. We provide specific examples of how deep learning may be applied in cancer diagnosis, prognosis and treatment management. We also assess the current limitations and challenges for the application of deep learning in precision oncology, including the lack of phenotypically rich data and the need for more explainable deep learning models. Finally, we conclude with a discussion of how current obstacles can be overcome to enable future clinical utilisation of deep learning.
Keywords: Artificial intelligence; Cancer genomics; Cancer of unknown primary; Deep learning; Explainability; Molecular subtypes; Multi-modal learning; Pharmacogenomics; Precision oncology; Prognosis; Tumour microenvironment.
© 2021. The Author(s).
Conflict of interest statement
John V Pearson and Nicola Waddell are co-founders and Board members of genomiQa. The remaining authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

