Immune imprinting and SARS-CoV-2 vaccine design
- PMID: 34580004
- PMCID: PMC8440232
- DOI: 10.1016/j.it.2021.09.001
Immune imprinting and SARS-CoV-2 vaccine design
Abstract
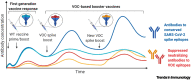
Reformulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines with variant strains is being pursued to combat the global surge in infections. We hypothesize that this may be suboptimal due to immune imprinting from earlier vaccination or infection with the original SARS-CoV-2 strain. New strategies may be needed to improve efficacy of SARS-CoV-2 variant vaccines.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
References
-
- Khoury D.S., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. - PubMed
-
- Amanat F., et al. The plasmablast response to SARS-CoV-2 mRNA vaccination is dominated by non-neutralizing antibodies that target both the NTD and the RBD. medRxiv. 2021 doi: 10.1101/2021.03.07.21253098. Published online May 1, 2021. - DOI
-
- Wu K., et al. Preliminary analysis of safety and immunogenicity of a SARS-CoV-2 variant vaccine booster. medRxiv. 2021 doi: 10.1101/2021.05.05.21256716. Published online May 6, 2021. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

