Tumor Immunology and Immunotherapy of Non-Small-Cell Lung Cancer
- PMID: 34580079
- PMCID: PMC8957639
- DOI: 10.1101/cshperspect.a037895
Tumor Immunology and Immunotherapy of Non-Small-Cell Lung Cancer
Abstract
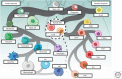
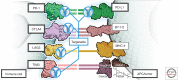
Historically, non-small-cell lung cancer (NSCLC) has been regarded as a nonimmunogenic tumor; however, recent studies have shown that NSCLCs are among the most responsive cancers to monoclonal antibody immune checkpoint inhibitors (ICIs). ICIs have dramatically improved clinical outcomes for a subset of patients (∼20%) with locally advanced and metastatic NSCLC, and they have also demonstrated promise as neoadjuvant therapy for early-stage resectable disease. Nevertheless, the majority of patients with NSCLC are refractory to ICIs for reasons that are poorly understood. Thus, major questions are: how do we initially identify the patients most likely to derive significant clinical benefit from these therapies; how can we increase the number of patients benefiting; what are the mechanisms of primary and acquired resistance to immune-based therapies; are there additional immune checkpoints besides PD-1/PD-L1 and CTLA-4 that can be targeted to provide greater clinical benefit to patients; and how do we best combine ICI therapy with surgery, radiotherapy, chemotherapy, and targeted therapy? To answer these questions, we need to deploy the latest technologies to study tumors and their microenvironment and how they interact with components of the innate and adaptive immune systems. There is also a need for new preclinical model systems to investigate the molecular mechanisms of resistance to treatment and identify novel therapeutic targets. Recent advances in technology are beginning to shed new light on the immune landscape of NSCLC that may uncover biomarkers of response and maximize the clinical benefit of immune-based therapies. Identification of the mechanisms of resistance should lead to the identification of novel targets and the generation of new therapeutic strategies that improve outcomes for a greater number of patients. In the sections below, we discuss the results of studies examining the immune microenvironment in NSCLC, summarize the clinical experience with immunotherapy for NSCLC, and review candidate biomarkers of response to these agents in NSCLC.
Copyright © 2022 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Abbosh C, Frankell A, Garnett A, Harrison T, Weichert M, Licon A, et al. 2020. Abstract CT023: phylogenetic tracking and minimal residual disease detection using ctDNA in early-stage NSCLC: A lung TRACERx study. Cancer Res 80: CT023-CT.
-
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. 2013. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3: 1355–1363. 10.1158/2159-8290.CD-13-0310 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
